Capturing pluripotency - PubMed (original) (raw)
Capturing pluripotency
Jose Silva et al. Cell. 2008.
Abstract
In this Essay, we argue that pluripotent epiblast founder cells in the embryo and embryonic stem (ES) cells in culture represent the ground state for a mammalian cell, signified by freedom from developmental specification or epigenetic restriction and capacity for autonomous self-replication. We speculate that cell-to-cell variation may be integral to the ES cell condition, safe-guarding self-renewal while continually presenting opportunities for lineage specification.
Figures
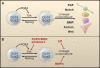
Figure 1
Maintaining Pluripotency Autoinductive FGF4/Erk signaling poises embryonic stem (ES) cells for lineage entry and must be resisted to allow self-renewal. (A) Oct4 and Sox2 direct expression of fgf4 and poise ES cells for lineage commitment. Elevated Erk activity provides a signal that renders pluripotent cells susceptible to lineage inductive cues. (B) Self-renewal of the pluripotent ES cell state requires overcoming the FGF4/Erk signal. The actions of FGF can be (1) blocked by selective pharmacological inhibitors of the FGF receptor (FGFR) and of Mek; (2) reversed by constitutive expression of Nanog; (3) counteracted by blockade of commitment effectors by the cytokine leukemia inhibitory factor (LIF) and the morphogen BMP4.
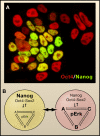
Figure 2
A Metastable Coalition The transcription factor Nanog secures self-renewal of ES cells, and cell-to-cell variation creates the possibility for differentiation. (A) Embryonic stem (ES) cells are heterogeneous. Immunostaining shows highly variable levels of Nanog protein in Oct4-positive undifferentiated ES cells. (B) In our model, lineage-associated transcriptional circuits (A, B, and C) are maintained below threshold levels due to mutual antagonism and suppression by the three transcription factors Oct4, Sox2, and Nanog. A destabilized transitional state arises when downregulation of Nanog coincides with increased activation of Erk. Phosphorylated Erk (pErk) may activate inductive signaling pathways or directly promote lineage-affiliated transcriptional networks. The fluctuations in network activities generated by pErk confer an opportunity to establish a new stable cell state. However, if Nanog levels rise before commitment is effected, the actions of pErk are neutralized, the metastable ground state is restored, and the gate is closed. Photo courtesy of J. Silva and A. Smith.
Similar articles
- Tracking the embryonic stem cell transition from ground state pluripotency.
Kalkan T, Olova N, Roode M, Mulas C, Lee HJ, Nett I, Marks H, Walker R, Stunnenberg HG, Lilley KS, Nichols J, Reik W, Bertone P, Smith A. Kalkan T, et al. Development. 2017 Apr 1;144(7):1221-1234. doi: 10.1242/dev.142711. Epub 2017 Feb 7. Development. 2017. PMID: 28174249 Free PMC article. - Suppression of Erk signalling promotes ground state pluripotency in the mouse embryo.
Nichols J, Silva J, Roode M, Smith A. Nichols J, et al. Development. 2009 Oct;136(19):3215-22. doi: 10.1242/dev.038893. Epub 2009 Aug 26. Development. 2009. PMID: 19710168 Free PMC article. - The epigenetic basis for embryonic stem cell pluripotency.
Szutorisz H, Dillon N. Szutorisz H, et al. Bioessays. 2005 Dec;27(12):1286-93. doi: 10.1002/bies.20330. Bioessays. 2005. PMID: 16299767 - The Art of Capturing Pluripotency: Creating the Right Culture.
Ying QL, Smith A. Ying QL, et al. Stem Cell Reports. 2017 Jun 6;8(6):1457-1464. doi: 10.1016/j.stemcr.2017.05.020. Stem Cell Reports. 2017. PMID: 28591647 Free PMC article. Review. - Molecular mechanisms involved in self-renewal and pluripotency of embryonic stem cells.
Liu N, Lu M, Tian X, Han Z. Liu N, et al. J Cell Physiol. 2007 May;211(2):279-86. doi: 10.1002/jcp.20978. J Cell Physiol. 2007. PMID: 17195167 Review.
Cited by
- Embryonic stem cell-derived trophoblast differentiation: a comparative review of the biology, function, and signaling mechanisms.
Giakoumopoulos M, Golos TG. Giakoumopoulos M, et al. J Endocrinol. 2013 Feb 25;216(3):R33-45. doi: 10.1530/JOE-12-0433. Print 2013 Mar. J Endocrinol. 2013. PMID: 23291503 Free PMC article. Review. - Global DNA hypomethylation prevents consolidation of differentiation programs and allows reversion to the embryonic stem cell state.
Schmidt CS, Bultmann S, Meilinger D, Zacher B, Tresch A, Maier KC, Peter C, Martin DE, Leonhardt H, Spada F. Schmidt CS, et al. PLoS One. 2012;7(12):e52629. doi: 10.1371/journal.pone.0052629. Epub 2012 Dec 27. PLoS One. 2012. PMID: 23300728 Free PMC article. - The many roads to Rome: induction of neural precursor cells from fibroblasts.
Lujan E, Wernig M. Lujan E, et al. Curr Opin Genet Dev. 2012 Oct;22(5):517-22. doi: 10.1016/j.gde.2012.07.002. Epub 2012 Aug 4. Curr Opin Genet Dev. 2012. PMID: 22868177 Free PMC article. Review. - Mechanisms of transcriptional precision in animal development.
Lagha M, Bothma JP, Levine M. Lagha M, et al. Trends Genet. 2012 Aug;28(8):409-16. doi: 10.1016/j.tig.2012.03.006. Epub 2012 Apr 16. Trends Genet. 2012. PMID: 22513408 Free PMC article. Review. - Towards human organ generation using interspecies blastocyst complementation: Challenges and perspectives for therapy.
Sarmah H, Sawada A, Hwang Y, Miura A, Shimamura Y, Tanaka J, Yamada K, Mori M. Sarmah H, et al. Front Cell Dev Biol. 2023 Jan 19;11:1070560. doi: 10.3389/fcell.2023.1070560. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36743411 Free PMC article. Review.
References
- Anton R., Kestler H.A., Kuhl M. FEBS Lett. 2007;581:5247–5254. - PubMed
- Brons I.G., Smithers L.E., Trotter M.W., Rugg-Gunn P., Sun B., Chuva de Sousa Lopes S.M., Howlett S.K., Clarkson A., Ahrlund-Richter L., Pedersen R.A., Vallier L. Nature. 2007;448:191–195. - PubMed
- Chambers I., Smith A. Oncogene. 2004;23:7150–7160. - PubMed
- Chambers I., Colby D., Robertson M., Nichols J., Lee S., Tweedie S., Smith A. Cell. 2003;113:643–655. - PubMed
- Chambers I., Silva J., Colby D., Nichols J., Nijmeijer B., Robertson M., Vrana J., Jones K., Grotewold L., Smith A. Nature. 2007;450:1230–1234. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources