Functional coactivation map of the human brain - PubMed (original) (raw)
Meta-Analysis
. 2008 Nov;18(11):2553-9.
doi: 10.1093/cercor/bhn014. Epub 2008 Feb 21.
Affiliations
- PMID: 18296434
- PMCID: PMC2567424
- DOI: 10.1093/cercor/bhn014
Meta-Analysis
Functional coactivation map of the human brain
Roberto Toro et al. Cereb Cortex. 2008 Nov.
Abstract
Understanding the interactions among different brain regions is fundamental to our understanding of brain function. Here we describe a complete map of functional connections in the human brain derived by an automatic meta-analysis of 825 neuroimaging articles, representing 3402 experiments. The likelihood of a functional connection between regions was estimated by studying the interdependence of their "activity," as reported in each experiment, across all experiments. We obtained a dense coactivation map that recovers some fundamental principles of the brain's functional connectivity, such as the symmetric interhemispheric connections, and important functional networks, such as the fronto-parietal attention network, the resting state network and the motor network.
Figures
Figure 1.
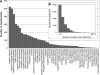
Characterization of the experiments used in the coactivation map. Distribution of the different cognitive domains represented by the experiments after the BrainMap classification (A). Histogram of the number of locations per experiment (B). Experiments reported on average 8 locations, and a decreasing number of experiments reported large numbers of locations.
Figure 2.
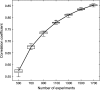
Reproducibility of the coactivation map. Pairs of partial coactivation maps computed from disjoint random subsets of the total database of experiments were progressively more similar as the number of experiments increased. The plot shows the distribution of the correlation coefficient for 20 pairs of partial coactivation maps computed from independent sets of 500, 700, 900, 1100, 1300, 1500, and 1700 experiments.
Figure 3.
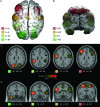
Symmetric interhemispheric coactivations. Coactivations of regions in the left hemisphere included most of the time the symmetric region in the right hemisphere, and vice versa. The figure shows 3-dimensional reconstructions (A, B) and stereotaxic slices of 4 networks corresponding to 4 seed-voxels in the axial plane z = 28 (C), and 4 networks in the coronal plane y = −6 (D). The network clusters are isosurfaces for P = 0.01, and the location of the seed-voxels is indicated by white squares in the stereotaxic slices.
Figure 4.
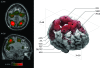
Fronto-parietal “attention” network. Three-dimensional reconstruction and axial (z = 48) and para-sagittal (x = 30) stereotaxic slices of the network recovered with a seed-voxel at the left intraparietal sulcus (IPS, x = −26, y = −58, z = 48). It includes the supplementary motor area (SMA) and preSMA, left and right anterior insula (aIns), frontal eye fields (FEF), dorsolateral prefrontal cortex (DLPFC), inferior precentral sulcus (iPCS), ventral occipital cortex (vOC), inferior parietal lobule (iPL), and the ventral IPS (vIPS). The network clusters are isosurfaces for P = 0.01, and the location of the seed-voxel is indicated in the axial slice by a white square.
Figure 5.
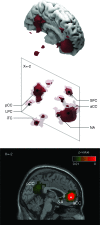
Cingulo-parietal “resting state” network. Three-dimensional reconstructions and sagittal stereotaxic slice (x = −2) of the network recovered with a seed-voxel at the anterior cingulate cortex (aCC, x = −2, y = 46, z = −4). It includes the posterior cingulate cortex (pCC), nucleus accumbens (NA), lateral parietal cortex (LPC), inferior temporal cortex (iTC), and the superior frontal cortex (SFC). The network clusters are isosurfaces for P = 0.01 (strong red), and P = 0.5 (in transparency). The location of the seed-voxel is indicated by a white square in the sagittal slice.
Figure 6.
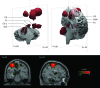
Cortico-diencephalo-cerebellar “motor” network. Three-dimensional reconstructions and coronal (y = −26) and para-sagittal (x = −34) stereotaxic slices of the network recovered with a seed-voxel at the dorsal part of the left central sulcus (CS, x = −34, y = −26, z = 60). The network includes the right central sulcus, caudal cingulate motor area (CMA), ipsilateral putamen (Pu), thalamus (Th), and left cerebellum (Cb-L), and the contralateral anterior lobe of the cerebellum (aCb). The network clusters are isosurfaces for P = 0.01, and the seed-voxel is indicated by white squares in the coronal and sagittal slices.
Similar articles
- Heterogeneous fractionation profiles of meta-analytic coactivation networks.
Laird AR, Riedel MC, Okoe M, Jianu R, Ray KL, Eickhoff SB, Smith SM, Fox PT, Sutherland MT. Laird AR, et al. Neuroimage. 2017 Apr 1;149:424-435. doi: 10.1016/j.neuroimage.2016.12.037. Epub 2017 Feb 20. Neuroimage. 2017. PMID: 28222386 Free PMC article. - Changes in structural and functional connectivity among resting-state networks across the human lifespan.
Betzel RF, Byrge L, He Y, Goñi J, Zuo XN, Sporns O. Betzel RF, et al. Neuroimage. 2014 Nov 15;102 Pt 2:345-57. doi: 10.1016/j.neuroimage.2014.07.067. Epub 2014 Aug 7. Neuroimage. 2014. PMID: 25109530 - Dynamic changes in large-scale functional network organization during autobiographical memory retrieval.
Inman CS, James GA, Vytal K, Hamann S. Inman CS, et al. Neuropsychologia. 2018 Feb;110:208-224. doi: 10.1016/j.neuropsychologia.2017.09.020. Epub 2017 Sep 23. Neuropsychologia. 2018. PMID: 28951163 - A dual-networks architecture of top-down control.
Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. Dosenbach NU, et al. Trends Cogn Sci. 2008 Mar;12(3):99-105. doi: 10.1016/j.tics.2008.01.001. Epub 2008 Feb 11. Trends Cogn Sci. 2008. PMID: 18262825 Free PMC article. Review. - The human connectome: a complex network.
Sporns O. Sporns O. Ann N Y Acad Sci. 2011 Apr;1224:109-125. doi: 10.1111/j.1749-6632.2010.05888.x. Epub 2011 Jan 4. Ann N Y Acad Sci. 2011. PMID: 21251014 Review.
Cited by
- Review of the Brain's Behaviour after Injury and Disease for Its Application in an Agent-Based Model (ABM).
Irastorza-Valera L, Soria-Gómez E, Benitez JM, Montáns FJ, Saucedo-Mora L. Irastorza-Valera L, et al. Biomimetics (Basel). 2024 Jun 14;9(6):362. doi: 10.3390/biomimetics9060362. Biomimetics (Basel). 2024. PMID: 38921242 Free PMC article. Review. - Neuroanatomical correlates of peripersonal space: bridging the gap between perception, action, emotion and social cognition.
Basile GA, Tatti E, Bertino S, Milardi D, Genovese G, Bruno A, Muscatello MRA, Ciurleo R, Cerasa A, Quartarone A, Cacciola A. Basile GA, et al. Brain Struct Funct. 2024 Jun;229(5):1047-1072. doi: 10.1007/s00429-024-02781-9. Epub 2024 Apr 29. Brain Struct Funct. 2024. PMID: 38683211 Free PMC article. Review. - Reduced homotopic interhemispheric connectivity in psychiatric disorders: evidence for both transdiagnostic and disorder specific features.
Yao S, Kendrick KM. Yao S, et al. Psychoradiology. 2022 Nov 24;2(4):129-145. doi: 10.1093/psyrad/kkac016. eCollection 2022 Dec. Psychoradiology. 2022. PMID: 38665271 Free PMC article. Review. - Altered spontaneous brain activity in lumbar disc herniation patients: insights from an ALE meta-analysis of neuroimaging data.
Qiu Z, Zhong X, Yang Q, Shi X, He L, Zhou H, Xu X. Qiu Z, et al. Front Neurosci. 2024 Feb 6;18:1349512. doi: 10.3389/fnins.2024.1349512. eCollection 2024. Front Neurosci. 2024. PMID: 38379762 Free PMC article. - A personalized cortical atlas for functional regions of interest.
Molloy MF, Osher DE. Molloy MF, et al. J Neurophysiol. 2023 Nov 1;130(5):1067-1080. doi: 10.1152/jn.00108.2023. Epub 2023 Sep 20. J Neurophysiol. 2023. PMID: 37727907 Free PMC article.
References
- Braitenberg V, Schüz A. Anatomy of the cortex: statistics and geometry. Berlin: Springer; 1991.
- Bressler SL, Tognoli E. Operational principles of neurocognitive networks. Int J Psychophysiol. 2006;60:139–148. - PubMed
- Brett M. 1999. The MNI brain and the Talairach atlas.
- Brett M, Johnsrude I, Owen A. The problem of localization in the human brain. Nat Rev Neurosci. 2002;3:243–249. - PubMed
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in primate cerebral cortex. Cereb Cortex. 1991;1:1–47. - PubMed