Pilot study of mutant ras peptide-based vaccine as an adjuvant treatment in pancreatic and colorectal cancers - PubMed (original) (raw)
Clinical Trial
Pilot study of mutant ras peptide-based vaccine as an adjuvant treatment in pancreatic and colorectal cancers
Antoun Toubaji et al. Cancer Immunol Immunother. 2008 Sep.
Abstract
Introduction: There is mounting evidence describing the immunosuppressive role of bulky metastatic disease, thus countering the therapeutic effects of tumor vaccine. Therefore, adjuvant immunotherapy may have a better impact on clinical outcome. In this phase II clinical trial, we aimed to test the feasibility of using a specific mutant ras peptide vaccine as an adjuvant immunotherapy in pancreatic and colorectal cancer patients.
Materials and methods: Twelve patients with no evidence of disease (NED), five pancreatic and seven colorectal cancer patients were vaccinated subcutaneously with 13-mer mutant ras peptide, corresponding to their tumor's ras mutation. Vaccinations were given every 4 weeks, up to a total of six vaccines.
Results: No serious acute or delayed systemic side effects were seen. We detected specific immune responses to the relevant mutant ras peptide by measuring IFN-gamma mRNA expression by quantitative real-time PCR. Five out of eleven patients showed a positive immune response. Furthermore, the five pancreatic cancer patients have shown a mean disease-free survival (DFS) of 35.2+ months and a mean overall survival (OS) of 44.4+ months. The seven colorectal cancer patients have shown a mean disease-free survival (DFS) of 27.2+ months and a mean overall survival (OS) of 41.5+ months.
Conclusion: In this study, we found that it is feasible to use mutant ras vaccine in the adjuvant setting. This vaccine is safe, can induce specific immune responses, and it appears to have a positive outcome in overall survival. Therefore, we believe that such an approach warrants further investigation in combination with other therapies.
Figures
Fig. 1
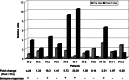
Immune response against the 13-mer corresponding mutant ras. q r-t PCR on PBMCs collected before vaccination (Pre-Vax) and after three vaccinations (Post-3Vax). Y axis values represent ratio of absolute IFN-γ mRNA copy numbers obtained by mutant ras peptide stimulation relative to wild type ras peptide stimulation, normalized with b-actin. Plus symbol indicates twofold increase or more of IFN-γ mRNA copies post-vaccination
Fig. 2
Disease free survival. Kaplan–Meier, estimated DFS of patients with immune response compared to patients with no immune response. P values were assessed using the exact long-rank test. DFS, disease free survival—calculated as time from the first vaccination until evidence of disease progression. Vertical lines indicate the patient is still alive at last assessment
Fig. 3
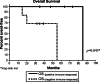
Overall survival. Kaplan–Meier, estimated OS of patients with immune response compared to patients with no immune response. P values were assessed using the exact long-rank test. OS, overall survival—calculated as time from the first vaccination until death. Vertical lines indicate the patient is still alive at last assessment
Similar articles
- Targeting mutated K-ras in pancreatic adenocarcinoma using an adjuvant vaccine.
Abou-Alfa GK, Chapman PB, Feilchenfeldt J, Brennan MF, Capanu M, Gansukh B, Jacobs G, Levin A, Neville D, Kelsen DP, O'Reilly EM. Abou-Alfa GK, et al. Am J Clin Oncol. 2011 Jun;34(3):321-5. doi: 10.1097/COC.0b013e3181e84b1f. Am J Clin Oncol. 2011. PMID: 20686403 - Mutated Ras peptides as vaccines in immunotherapy of cancer.
Gjertsen MK, Gaudernack G. Gjertsen MK, et al. Vox Sang. 1998;74 Suppl 2:489-95. doi: 10.1111/j.1423-0410.1998.tb05462.x. Vox Sang. 1998. PMID: 9704487 Review. - The immunological and clinical effects of mutated ras peptide vaccine in combination with IL-2, GM-CSF, or both in patients with solid tumors.
Rahma OE, Hamilton JM, Wojtowicz M, Dakheel O, Bernstein S, Liewehr DJ, Steinberg SM, Khleif SN. Rahma OE, et al. J Transl Med. 2014 Feb 24;12:55. doi: 10.1186/1479-5876-12-55. J Transl Med. 2014. PMID: 24565030 Free PMC article. Clinical Trial. - Phase II clinical trial using novel peptide cocktail vaccine as a postoperative adjuvant treatment for surgically resected pancreatic cancer patients.
Miyazawa M, Katsuda M, Maguchi H, Katanuma A, Ishii H, Ozaka M, Yamao K, Imaoka H, Kawai M, Hirono S, Okada KI, Yamaue H. Miyazawa M, et al. Int J Cancer. 2017 Feb 15;140(4):973-982. doi: 10.1002/ijc.30510. Epub 2016 Nov 21. Int J Cancer. 2017. PMID: 27861852 Clinical Trial.
Cited by
- Development of a cytokine-modified allogeneic whole cell pancreatic cancer vaccine.
Laheru D, Biedrzycki B, Jaffee EM. Laheru D, et al. Methods Mol Biol. 2013;980:175-203. doi: 10.1007/978-1-62703-287-2_9. Methods Mol Biol. 2013. PMID: 23359154 Free PMC article. - Anti-EGFR therapy in metastatic colorectal cancer: mechanisms and potential regimens of drug resistance.
Li QH, Wang YZ, Tu J, Liu CW, Yuan YJ, Lin R, He WL, Cai SR, He YL, Ye JN. Li QH, et al. Gastroenterol Rep (Oxf). 2020 Jun 23;8(3):179-191. doi: 10.1093/gastro/goaa026. eCollection 2020 Jun. Gastroenterol Rep (Oxf). 2020. PMID: 32665850 Free PMC article. Review. - The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research.
Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, Mellman I, Prindiville SA, Viner JL, Weiner LM, Matrisian LM. Cheever MA, et al. Clin Cancer Res. 2009 Sep 1;15(17):5323-37. doi: 10.1158/1078-0432.CCR-09-0737. Clin Cancer Res. 2009. PMID: 19723653 Free PMC article. - Immunotherapy and Prevention of Pancreatic Cancer.
Morrison AH, Byrne KT, Vonderheide RH. Morrison AH, et al. Trends Cancer. 2018 Jun;4(6):418-428. doi: 10.1016/j.trecan.2018.04.001. Epub 2018 Apr 30. Trends Cancer. 2018. PMID: 29860986 Free PMC article. Review. - Immunotherapy in pancreatic adenocarcinoma-overcoming barriers to response.
Rosenberg A, Mahalingam D. Rosenberg A, et al. J Gastrointest Oncol. 2018 Feb;9(1):143-159. doi: 10.21037/jgo.2018.01.13. J Gastrointest Oncol. 2018. PMID: 29564181 Free PMC article. Review.
References
- Hruban RH, van Mansfeld AD, Offerhaus GJ, van Weering DH, Allison DC, Goodman SN, et al. K-ras oncogene activation in adenocarcinoma of the human pancreas. A study of 82 carcinomas using a combination of mutant-enriched polymerase chain reaction analysis and allele-specific oligonucleotide hybridization. Am J Pathol. 1993;143(2):545–554. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous