Raves and risks for erythropoietin - PubMed (original) (raw)
Review
Raves and risks for erythropoietin
Kenneth Maiese et al. Cytokine Growth Factor Rev. 2008 Apr.
Abstract
Global use of erythropoietin (EPO) continues to increase as a proven agent for the treatment of anemia. Yet, EPO is no longer believed to have exclusive biological activity in the hematopoietic system and is now considered applicable for a variety of disorders such as diabetes, Alzheimer's disease, and cardiovascular disease. Treatment with EPO is considered to be robust and can prevent metabolic compromise, neuronal and vascular degeneration, and inflammatory cell activation. On the converse side, observations that EPO administration is not without risk have fueled controversy. Here we present recent advances that have elucidated a number of novel cellular pathways governed by EPO to open new therapeutic avenues for this agent and avert its potential deleterious effects.
Figures
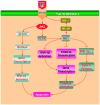
Figure 1. Cytoprotection by erythropoietin (EPO) requires multiple signal transduction pathways
EPO and the EPO receptor (EPOR) can increase cell survival, promote progenitor cell development, and control inflammatory cell activation through pathways that involve the Janus-tyrosine kinase 2 (Jak2) protein, protein kinase B (Akt), and signal transducer and activator of transcription (STAT) proteins. Subsequent downstream signaling governs extracellular signal-related kinases (ERKs), the forkhead family member FOXO3a, glycogen synthase kinase-3β (GSK-3β), and nuclear factor-κB (NF-κB). Intimately linked to the ability of EPO to maintain cellular integrity and prevent inflammatory activation that ultimately can lead to cellular apoptosis are the maintenance of mitochondrial membrane potential (ΔΨm), the release of cytochrome c, (Cyto-c), and the prevention of caspase activation.
Figure 2. Erythropoietin (EPO) sequesters FOXO3a in the cytoplasm during oxygen-glucose deprivation (OGD)
Administration of EPO (10 ng/ml) with an 8 hour period of OGD, OGD alone, or untreated cells (Control) was followed at 6 hours with immunofluorescent staining for FOXO3a (Texas-red) in endothelial cells (ECs). Nuclei of ECs were counterstained with DAPI. In merged images, cells with combined EPO and OGD show EC nuclei with minimal FOXO3a staining (blue/white) and show EC cytoplasm with significant FOXO3a staining (red) in contrast to cells with OGD alone with significant FOXO3a staining in both the cytoplasm and the nuclei of ECs, demonstrating the ability of EPO to sequester FOXO3a in the cytoplasm.
Similar articles
- Erythropoietin: elucidating new cellular targets that broaden therapeutic strategies.
Maiese K, Chong ZZ, Li F, Shang YC. Maiese K, et al. Prog Neurobiol. 2008 Jun;85(2):194-213. doi: 10.1016/j.pneurobio.2008.02.002. Epub 2008 Mar 4. Prog Neurobiol. 2008. PMID: 18396368 Free PMC article. Review. - New avenues of exploration for erythropoietin.
Maiese K, Li F, Chong ZZ. Maiese K, et al. JAMA. 2005 Jan 5;293(1):90-5. doi: 10.1001/jama.293.1.90. JAMA. 2005. PMID: 15632341 Free PMC article. Review. - Erythropoietin requires NF-kappaB and its nuclear translocation to prevent early and late apoptotic neuronal injury during beta-amyloid toxicity.
Chong ZZ, Li F, Maiese K. Chong ZZ, et al. Curr Neurovasc Res. 2005 Dec;2(5):387-99. doi: 10.2174/156720205774962683. Curr Neurovasc Res. 2005. PMID: 16375720 Free PMC article. - Erythropoietin: a hormone with multiple functions.
Lombardero M, Kovacs K, Scheithauer BW. Lombardero M, et al. Pathobiology. 2011;78(1):41-53. doi: 10.1159/000322975. Epub 2011 Apr 5. Pathobiology. 2011. PMID: 21474975 Review. - Erythropoietin and the vascular wall: the controversy continues.
Jelkmann W, Elliott S. Jelkmann W, et al. Nutr Metab Cardiovasc Dis. 2013 Dec;23 Suppl 1:S37-43. doi: 10.1016/j.numecd.2012.04.002. Epub 2012 Jun 7. Nutr Metab Cardiovasc Dis. 2013. PMID: 22682530 Review.
Cited by
- Dual Delivery of EPO and BMP2 from a Novel Modular Poly-ɛ-Caprolactone Construct to Increase the Bone Formation in Prefabricated Bone Flaps.
Patel JJ, Modes JE, Flanagan CL, Krebsbach PH, Edwards SP, Hollister SJ. Patel JJ, et al. Tissue Eng Part C Methods. 2015 Sep;21(9):889-97. doi: 10.1089/ten.TEC.2014.0643. Epub 2015 Jul 22. Tissue Eng Part C Methods. 2015. PMID: 25809081 Free PMC article. - Nicotinamide as a Foundation for Treating Neurodegenerative Disease and Metabolic Disorders.
Maiese K. Maiese K. Curr Neurovasc Res. 2021;18(1):134-149. doi: 10.2174/1567202617999210104220334. Curr Neurovasc Res. 2021. PMID: 33397266 Free PMC article. Review. - Rogue proliferation versus restorative protection: where do we draw the line for Wnt and forkhead signaling?
Maiese K, Chong ZZ, Shang YC, Hou J. Maiese K, et al. Expert Opin Ther Targets. 2008 Jul;12(7):905-16. doi: 10.1517/14728222.12.7.905. Expert Opin Ther Targets. 2008. PMID: 18554157 Free PMC article. Review. - Erythropoietin, forkhead proteins, and oxidative injury: biomarkers and biology.
Maiese K, Hou J, Chong ZZ, Shang YC. Maiese K, et al. ScientificWorldJournal. 2009 Oct 2;9:1072-104. doi: 10.1100/tsw.2009.121. ScientificWorldJournal. 2009. PMID: 19802503 Free PMC article. Review. - The cytokine receptor CRLF3 is a human neuroprotective EV-3 (Epo) receptor.
Knorr DY, Rodriguez Polo I, Pies HS, Schwedhelm-Domeyer N, Pauls S, Behr R, Heinrich R. Knorr DY, et al. Front Mol Neurosci. 2023 Apr 6;16:1154509. doi: 10.3389/fnmol.2023.1154509. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37168680 Free PMC article.
References
- Carnot P, DeFlandre C. Sur l’activite hemopoietique de serum au cours de la regeneration du sang. C R Acad Sci (Paris) 1906;143:384–386.
- Maiese K, Li F, Chong ZZ. Erythropoietin in the brain: can the promise to protect be fulfilled? Trends Pharmacol Sci. 2004;25:577–583. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials