Hypothalamic neurodegeneration and adult-onset obesity in mice lacking the Ubb polyubiquitin gene - PubMed (original) (raw)
Hypothalamic neurodegeneration and adult-onset obesity in mice lacking the Ubb polyubiquitin gene
Kwon-Yul Ryu et al. Proc Natl Acad Sci U S A. 2008.
Abstract
Nearly all neurodegenerative diseases are associated with abnormal accumulation of ubiquitin (Ub) conjugates within neuronal inclusion bodies. To directly test the hypothesis that depletion of cellular Ub is sufficient to cause neurodegeneration, we have disrupted Ubb, one of four genes that supply Ub in the mouse. Here, we report that loss of Ubb led to a progressive degenerative disorder affecting neurons within the arcuate nucleus of the hypothalamus. This neurodegenerative cytopathology was accompanied by impaired hypothalamic control of energy balance and adult-onset obesity. Ubb was highly expressed in vulnerable hypothalamic neurons and total Ub levels were selectively reduced in the hypothalamus of Ubb-null mice. These findings demonstrate that maintenance of adequate supplies of cellular Ub is essential for neuronal survival and establish that decreased Ub availability is sufficient to cause neuronal dysfunction and death.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
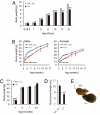
Fig. 1.
Abnormal growth of _Ubb_−/− mice. (A) Newborn _Ubb_−/− (−/−) (n = 18) mice are smaller and exhibit reduced perinatal weight gain compared with littermate controls (+/+ and +/−) (n = 39). Smaller size of _Ubb_−/− mice is also evident at E18.5 (+/+ and +/−, n = 13; −/−, n = 3). (B) Growth curve of male and female mice after weaning. Body weights of _Ubb_−/− (male, n = 19; female, n = 17) mice are significantly lower than those of their littermate controls (male, n = 51; female, n = 76) before 16 weeks of age. At 16 weeks of age, there is no significant difference in weight between _Ubb_−/− mice and littermate controls. (C) Total body mass of male mice determined by DEXA (+/+ and +/−, n = 4–12; −/−, n = 3 or 4). (D) Snout–anus length of 4-month-old mice (+/+ and +/−, n = 18; −/−, n = 13). (E) Representative photographs of adult Ubb+/+ (+/+) and _Ubb_−/− littermates. All data are expressed as means ± SEM from the indicated number of mice. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001 for _Ubb_−/− vs. littermate controls.
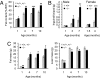
Fig. 2.
Altered body composition in adult _Ubb_−/− mice. (A) Whole-body fat content of male _Ubb_−/− mice (n = 3 or 4) and littermate controls (n = 4–12) measured by DEXA. Fat content is expressed as a percentage of body weight. (B) Inguinal fat pad weight of male (+/+, n = 6–11; −/−, n = 6–11) and female (+/+, n = 5 or 7; −/−, n = 6) mice. (C) Fat and lean mass of male _Ubb_−/− mice (n = 3 or 4) and littermate controls (n = 4–12) measured by DEXA. All data are expressed as means ± SEM from the indicated number of mice. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001 for _Ubb_−/− vs. littermate controls or Ubb+/+.
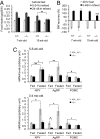
Fig. 3.
Defective central regulation of body weight and energy homeostasis in _Ubb_−/− mice. (A) Ad libitum food intake during the 24 h preceding fasting for 48 h and the two 24-h intervals after refeeding was measured and expressed as per gram body weight in 7-week-old (+/+, n = 9; −/−, n = 11) and 15-week-old (+/+, n = 11; −/−, n = 11) mice. (B) Recovery of body weight after fasting for 48 h and refeeding for 48 h in 7-week-old (+/+, n = 9; −/−, n = 11) and 15-week-old (+/+, n = 11; −/−, n = 11) mice. Loss of body weight after fasting was arbitrarily assigned as −100% in each genotype of mice. (C) Abnormal expression of hypothalamic neuropeptides in adult _Ubb_−/− mice. Normalized mRNA levels, measured by quantitative real-time RT-PCR from hypothalamus of young (5–6 weeks old) and adult (3–4 months old) ad libitum fed (+/+ and +/−, n = 12; −/−, n = 5 or 6) or 48-h fasted (+/+ and +/−, n = 12 or 16; −/−, n = 6) mice. All data are expressed as means ± SEM from the indicated number of mice. In A and C: ∗, P < 0.05; ∗∗, P < 0.01; #, P = 0.07. In B: ∗∗∗, P < 0.001 vs. 15-week-old Ubb+/+ mice after refeeding for 48 h.
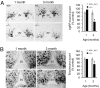
Fig. 4.
Degeneration of hypothalamic neurons in adult _Ubb_−/− mice. In situ hybridization of AgRP (A) and NSE (B) mRNA from _Ubb_−/− mice (n = 3 or 4) and littermate controls (n = 3 or 4). Images are representative for each age and group. For quantification, stereological counts were obtained from eight serial sections generated from each brain. 3V, third ventricle. The data are expressed as means ± SEM from the indicated number of mice. ∗, P < 0.05.
Fig. 5.
Dominant contribution of Ubb to Ub content of hypothalamus. (A) Quantification of ubiquitin transcript levels in hypothalamus of 3- to 4-month-old mice. (Left) Indicated mRNA levels, measured by quantitative real-time RT-PCR (+/+, n = 5; +/−, n = 6; −/−, n = 4). (Right) Relative contribution of ubiquitin genes to total Ub-coding potential normalized to the number of Ub moieties encoded by each ubiquitin transcript. The data are expressed as means ± SEM from the indicated number of mice. (B) Total Ub content in whole brain (n = 6 per genotype) and hypothalamus (+/+, n = 6; +/−, n = 3; −/−, n = 4) from 5- to 6-month-old mice. The data are expressed as means ± SEM from the indicated number of mice. ∗∗∗, P < 0.001 vs. Ubb+/+ mice. (C) Neuronal pattern of Ubb expression in the arcuate nucleus. Representative confocal images of GFP-Puror and Nissl stain from 4-month-old _Ubb_−/− mice (n = 3) are shown. The far right image shows the arcuate nucleus at higher magnification. VMH, ventromedial hypothalamus; ARC, arcuate nucleus; 3V, third ventricle. (Scale bars, 100 μm.) (D) Colocalization of NPY, AgRP, and α-MSH immunoreactive neurons in the arcuate nucleus to _Ubb_-expressing neurons. Representative confocal images of GFP-Puror and NPY, AgRP, and α-MSH immunoreactivity from 4-month-old Ubb+/− mice (n = 4) are shown. 3V, third ventricle. (Scale bars, 100 μm.)
Similar articles
- Locus coeruleus neurons are resistant to dysfunction and degeneration by maintaining free ubiquitin levels although total ubiquitin levels decrease upon disruption of polyubiquitin gene Ubb.
Park CW, Ryu HW, Ryu KY. Park CW, et al. Biochem Biophys Res Commun. 2012 Feb 17;418(3):541-6. doi: 10.1016/j.bbrc.2012.01.063. Epub 2012 Jan 21. Biochem Biophys Res Commun. 2012. PMID: 22285186 - Loss of polyubiquitin gene Ubb leads to metabolic and sleep abnormalities in mice.
Ryu KY, Fujiki N, Kazantzis M, Garza JC, Bouley DM, Stahl A, Lu XY, Nishino S, Kopito RR. Ryu KY, et al. Neuropathol Appl Neurobiol. 2010 Jun;36(4):285-99. doi: 10.1111/j.1365-2990.2009.01057.x. Epub 2009 Dec 8. Neuropathol Appl Neurobiol. 2010. PMID: 20002312 Free PMC article. - Restoration of cellular ubiquitin reverses impairments in neuronal development caused by disruption of the polyubiquitin gene Ubb.
Ryu HW, Park CW, Ryu KY. Ryu HW, et al. Biochem Biophys Res Commun. 2014 Oct 24;453(3):443-8. doi: 10.1016/j.bbrc.2014.09.103. Epub 2014 Oct 1. Biochem Biophys Res Commun. 2014. PMID: 25280998 - Review: unchained maladie - a reassessment of the role of Ubb(+1) -capped polyubiquitin chains in Alzheimer's disease.
Chadwick L, Gentle L, Strachan J, Layfield R. Chadwick L, et al. Neuropathol Appl Neurobiol. 2012 Apr;38(2):118-31. doi: 10.1111/j.1365-2990.2011.01236.x. Neuropathol Appl Neurobiol. 2012. PMID: 22082077 Review. - Regulation of polyubiquitin genes to meet cellular ubiquitin requirement.
Han SW, Jung BK, Ryu KY. Han SW, et al. BMB Rep. 2021 Apr;54(4):189-195. doi: 10.5483/BMBRep.2021.54.4.005. BMB Rep. 2021. PMID: 33612153 Free PMC article. Review.
Cited by
- The essential and downstream common proteins of amyotrophic lateral sclerosis: A protein-protein interaction network analysis.
Mao Y, Kuo SW, Chen L, Heckman CJ, Jiang MC. Mao Y, et al. PLoS One. 2017 Mar 10;12(3):e0172246. doi: 10.1371/journal.pone.0172246. eCollection 2017. PLoS One. 2017. PMID: 28282387 Free PMC article. - Distinct effects of ubiquitin overexpression on NMJ structure and motor performance in mice expressing catalytically inactive USP14.
Vaden JH, Watson JA, Howard AD, Chen PC, Wilson JA, Wilson SM. Vaden JH, et al. Front Mol Neurosci. 2015 Apr 23;8:11. doi: 10.3389/fnmol.2015.00011. eCollection 2015. Front Mol Neurosci. 2015. PMID: 25954152 Free PMC article. - The fallacy of ratio correction to address confounding factors.
Karp NA, Segonds-Pichon A, Gerdin AK, Ramírez-Solis R, White JK. Karp NA, et al. Lab Anim. 2012 Jul;46(3):245-52. doi: 10.1258/la.2012.012003. Lab Anim. 2012. PMID: 22829707 Free PMC article. - SUMO1-dependent modulation of SERCA2a in heart failure.
Kho C, Lee A, Jeong D, Oh JG, Chaanine AH, Kizana E, Park WJ, Hajjar RJ. Kho C, et al. Nature. 2011 Sep 7;477(7366):601-5. doi: 10.1038/nature10407. Nature. 2011. PMID: 21900893 Free PMC article. - Interplay between the Endogenous Opioid System and Proteasome Complex: Beyond Signaling.
Caputi FF, Rullo L, Stamatakos S, Candeletti S, Romualdi P. Caputi FF, et al. Int J Mol Sci. 2019 Mar 21;20(6):1441. doi: 10.3390/ijms20061441. Int J Mol Sci. 2019. PMID: 30901925 Free PMC article. Review.
References
- Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. - PubMed
- Hochstrasser M. Ubiquitin-dependent protein degradation. Annu Rev Genet. 1996;30:405–439. - PubMed
- D'Andrea A, Pellman D. Deubiquitinating enzymes: A new class of biological regulators. Crit Rev Biochem Mol Biol. 1998;33:337–352. - PubMed
- Ohtani-Kaneko R, et al. Nerve growth factor (NGF) induces increase in multi-ubiquitin chains and concomitant decrease in free ubiquitin in nuclei of PC12h. Neurosci Res. 1996;26:349–355. - PubMed
- Takada K, Hibi N, Tsukada Y, Shibasaki T, Ohkawa K. Ability of ubiquitin radioimmunoassay to discriminate between monoubiquitin and multi-ubiquitin chains. Biochim Biophys Acta. 1996;1290:282–288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 MH073844-05/MH/NIMH NIH HHS/United States
- R01 MH073844/MH/NIMH NIH HHS/United States
- R01 MH076929-02/MH/NIMH NIH HHS/United States
- R01 MH076929-04/MH/NIMH NIH HHS/United States
- R01 MH073844-04/MH/NIMH NIH HHS/United States
- R01 MH076929-01A1/MH/NIMH NIH HHS/United States
- R01 MH076929/MH/NIMH NIH HHS/United States
- R01 MH073844-03/MH/NIMH NIH HHS/United States
- R01 MH073844-01A2/MH/NIMH NIH HHS/United States
- R01 MH076929-05/MH/NIMH NIH HHS/United States
- R01 MH073844-02/MH/NIMH NIH HHS/United States
- R01 MH076929-03/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous