Loss of Bardet-Biedl syndrome proteins alters the morphology and function of motile cilia in airway epithelia - PubMed (original) (raw)
. 2008 Mar 4;105(9):3380-5.
doi: 10.1073/pnas.0712327105. Epub 2008 Feb 25.
Sara L Farmen, Thomas O Moninger, Thomas R Businga, Michael P Andrews, Kevin Bugge, Charles C Searby, Darryl Nishimura, Kim A Brogden, Joel N Kline, Val C Sheffield, Michael J Welsh
Affiliations
- PMID: 18299575
- PMCID: PMC2265193
- DOI: 10.1073/pnas.0712327105
Loss of Bardet-Biedl syndrome proteins alters the morphology and function of motile cilia in airway epithelia
Alok S Shah et al. Proc Natl Acad Sci U S A. 2008.
Abstract
Mutations in a group of genes that contribute to ciliary function cause Bardet-Biedl syndrome (BBS). Most studies of BBS have focused on primary, sensory cilia. Here, we asked whether loss of BBS proteins would also affect motile cilia lining the respiratory tract. We found that BBS genes were expressed in human airway epithelia, and BBS2 and BBS4 localized to cellular structures associated with motile cilia. Although BBS proteins were not required for ciliogenesis, their loss caused structural defects in a fraction of cilia covering mouse airway epithelia. The most common abnormality was bulges filled with vesicles near the tips of cilia. We discovered this same misshapen appearance in airway cilia from Bbs1, Bbs2, Bbs4, and Bbs6 mutant mice. The structural abnormalities were accompanied by functional defects; ciliary beat frequency was reduced in Bbs mutant mice. Previous reports suggested BBS might increase the incidence of asthma. However, compared with wild-type controls, neither airway hyperresponsiveness nor inflammation increased in Bbs2(-/-) or Bbs4(-/-) mice immunized with ovalbumin. Instead, these animals were partially protected from airway hyperresponsiveness. These results emphasize the role of BBS proteins in both the structure and function of motile cilia. They also invite additional scrutiny of motile cilia dysfunction in patients with this disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
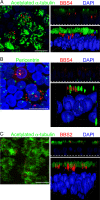
Fig. 1.
BBS4 and BBS2 localized to cilia and related structures in primary cultures of differentiated human airway epithelia. Airway epithelia were infected with Ad-3xFlag-tagged-BBS4 (A and B) or BBS2 (C) and immunostained 28 h later. Each image shows a stack of X-Y confocal images on the left, a single X-Z image in the upper right (the dashed line indicates the top of the filter on which epithelia grew), and a 3D surface projection in the lower right. (A) BBS4 (red) localized in multiple puncta beneath the cilia in the area of the basal body. Acetylated α-tubulin (green) is a marker of cilia (45). Nuclei were stained with DAPI (blue). (B) BBS4 (red) localized adjacent to or with pericentrin (green). Pericentrin staining occurred at the basal bodies in the apical portion of ciliated cells and near the nucleus of basal cells in centrioles (24). (C) BBS2 (red) localized in the region of cilia (acetylated α-tubulin, green) and the basal body. Control epithelia treated with secondary antibodies showed no staining (data not shown). (Scale bars: 10 μm.)
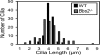
Fig. 2.
Loss of BBS2 changed the distribution of cilia length. Cilia length was measured by using light microscopy of H&E sections. _Bbs2_−/− and wild-type cilia showed the same average length: 4.1 μm. However, _Bbs2_−/− cilia exhibited a greater variation in length; variance for wild-type cilia (0.20) differed from _Bbs2_−/− cilia (1.22); P < 0.001, Levene test.
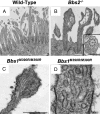
Fig. 3.
Loss of BBS protein produced abnormal morphology of motile cilia. Images are SEMs of airway epithelial cultures from wild-type (A), _Bbs2_−/− (B), and _Bbs4_−/− (C) mice and of the in vivo tracheal surface from wild-type (D), _Bbs2_−/− (E), and _Bbs4_−/− (F) mice. B shows cilia with bulges at their distal end and along the shaft. Some of the cilia show sprouts at their end, giving a glove-with-fingers-like appearance; also see Inset. B and C also show cup-shaped cilia. SEMs from in vivo tissue showed similar abnormalities but less frequently. We have never observed these abnormalities in wild-type epithelia. In addition to cilia, the apical surface contains microvilli of variable number and length. (Scale bars: 1 μm.)
Fig. 4.
Loss of BBS proteins caused a build up of vesicles in motile cilia. Data are TEM images from wild-type (A), Bbs2 null (B), and Bbs1 mutant (M390R) (C and D) primary cultures of differentiated airway epithelia. B shows bulges at the distal end of two cilia; the one on the right has a cup-like shape, and both contain vesicles. (Inset) Vesicles adjacent to an intact microtubule structure. D shows that the vesicles have a bilayer membrane. (Scale bars: A–C, 0.5 μm; D, 100 nm.)
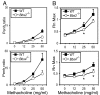
Fig. 5.
_Bbs2_−/− and _Bbs4_−/− mice did not have increased airway hyperresponsiveness. Mice were immunized with ovalbumin and challenged with methacholine. We studied _Bbs2_−/− and _Bbs4_−/− mice and their wild-type littermates. Airway hyperresponsiveness was measured noninvasively by using whole-body plethysmography and recording enhanced pause (Penh) (A) or by using an invasive small-animal ventilator (Flexivent) (B). The Bbs mice showed reduced airway hyperresponsiveness. n = 8 for _Bbs2_−/− and wild type; n = 6 for _Bbs4_−/− and wild type. Asterisks indicate P < 0.05.
Fig. 6.
Loss of BBS1 and BBS4 reduced ciliary beat frequency but did not alter mucociliary transport. (A) Data are ciliary beat frequency in differentiated airway epithelial cultures. n = 8 mice. Asterisks indicate P < 0.001. (B) Mucociliary transport rates did not significantly differ between wild-type and Bbs null mice. n = 6 mice. Data are mean ± SEM.
Similar articles
- Bardet Biedl syndrome: motile ciliary phenotype.
Shoemark A, Dixon M, Beales PL, Hogg CL. Shoemark A, et al. Chest. 2015 Mar;147(3):764-770. doi: 10.1378/chest.13-2913. Chest. 2015. PMID: 25317630 - Requirement of Bardet-Biedl syndrome proteins for leptin receptor signaling.
Seo S, Guo DF, Bugge K, Morgan DA, Rahmouni K, Sheffield VC. Seo S, et al. Hum Mol Genet. 2009 Apr 1;18(7):1323-31. doi: 10.1093/hmg/ddp031. Epub 2009 Jan 15. Hum Mol Genet. 2009. PMID: 19150989 Free PMC article. - Leptin resistance contributes to obesity and hypertension in mouse models of Bardet-Biedl syndrome.
Rahmouni K, Fath MA, Seo S, Thedens DR, Berry CJ, Weiss R, Nishimura DY, Sheffield VC. Rahmouni K, et al. J Clin Invest. 2008 Apr;118(4):1458-67. doi: 10.1172/JCI32357. J Clin Invest. 2008. PMID: 18317593 Free PMC article. - Bardet-Biedl syndrome: Is it only cilia dysfunction?
Novas R, Cardenas-Rodriguez M, Irigoín F, Badano JL. Novas R, et al. FEBS Lett. 2015 Nov 14;589(22):3479-91. doi: 10.1016/j.febslet.2015.07.031. Epub 2015 Jul 29. FEBS Lett. 2015. PMID: 26231314 Review. - Establishing a connection between cilia and Bardet-Biedl Syndrome.
Mykytyn K, Sheffield VC. Mykytyn K, et al. Trends Mol Med. 2004 Mar;10(3):106-9. doi: 10.1016/j.molmed.2004.01.003. Trends Mol Med. 2004. PMID: 15106604 Review.
Cited by
- Ectopic expression of human BBS4 can rescue Bardet-Biedl syndrome phenotypes in Bbs4 null mice.
Chamling X, Seo S, Bugge K, Searby C, Guo DF, Drack AV, Rahmouni K, Sheffield VC. Chamling X, et al. PLoS One. 2013;8(3):e59101. doi: 10.1371/journal.pone.0059101. Epub 2013 Mar 15. PLoS One. 2013. PMID: 23554981 Free PMC article. - Cycling of the signaling protein phospholipase D through cilia requires the BBSome only for the export phase.
Lechtreck KF, Brown JM, Sampaio JL, Craft JM, Shevchenko A, Evans JE, Witman GB. Lechtreck KF, et al. J Cell Biol. 2013 Apr 15;201(2):249-61. doi: 10.1083/jcb.201207139. J Cell Biol. 2013. PMID: 23589493 Free PMC article. - The blind leading the obese: the molecular pathophysiology of a human obesity syndrome.
Sheffield VC. Sheffield VC. Trans Am Clin Climatol Assoc. 2010;121:172-81; discussion 181-2. Trans Am Clin Climatol Assoc. 2010. PMID: 20697559 Free PMC article. Review. - Galectin 3 Deficiency Alters Chondrocyte Primary Cilium Formation and Exacerbates Cartilage Destruction via Mitochondrial Apoptosis.
Hafsia N, Forien M, Renaudin F, Delacour D, Reboul P, Van Lent P, Cohen-Solal M, Lioté F, Poirier F, Ea HK. Hafsia N, et al. Int J Mol Sci. 2020 Feb 22;21(4):1486. doi: 10.3390/ijms21041486. Int J Mol Sci. 2020. PMID: 32098291 Free PMC article. - Next-generation sequence analysis of genes associated with obesity and nonalcoholic fatty liver disease-related cirrhosis in extreme obesity.
Gerhard GS, Chu X, Wood GC, Gerhard GM, Benotti P, Petrick AT, Gabrielsen J, Strodel WE, Still CD, Argyropoulos G. Gerhard GS, et al. Hum Hered. 2013;75(2-4):144-51. doi: 10.1159/000351719. Epub 2013 Sep 27. Hum Hered. 2013. PMID: 24081230 Free PMC article.
References
- Mykytyn K, Sheffield VC. Establishing a connection between cilia and Bardet–Biedl syndrome. Trends Mol Med. 2004;10:106–109. - PubMed
- Nachury MV, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1213. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DE014390/DE/NIDCR NIH HHS/United States
- DE014390/DE/NIDCR NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- HL51670/HL/NHLBI NIH HHS/United States
- P50 HL061234/HL/NHLBI NIH HHS/United States
- R01 HL059324/HL/NHLBI NIH HHS/United States
- P30 DK054759/DK/NIDDK NIH HHS/United States
- T32 GM007337/GM/NIGMS NIH HHS/United States
- HL59324/HL/NHLBI NIH HHS/United States
- P01 HL051670/HL/NHLBI NIH HHS/United States
- HL61234/HL/NHLBI NIH HHS/United States
- DK54759/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases