Selective and shared contributions of the hippocampus and perirhinal cortex to episodic item and associative encoding - PubMed (original) (raw)
Selective and shared contributions of the hippocampus and perirhinal cortex to episodic item and associative encoding
Bernhard P Staresina et al. J Cogn Neurosci. 2008 Aug.
Abstract
Although the general role of the medial-temporal lobe (MTL) in episodic memory is well established, controversy surrounds the precise division of labor between distinct MTL subregions. The perirhinal cortex (PrC) has been hypothesized to support nonassociative item encoding that contributes to later familiarity, whereas the hippocampus supports associative encoding that selectively contributes to later recollection. However, because previous paradigms have predominantly used recollection of the item context as a measure of associative encoding, it remains unclear whether recollection of different kinds of episodic detail depends on the same or different MTL encoding operations. In our current functional magnetic resonance imaging study, we devised a subsequent memory paradigm that assessed successful item encoding in addition to the encoding of two distinct episodic details: an item-color and an item-context detail. Hippocampal encoding activation was selectively enhanced during trials leading to successful recovery of either an item-color or item-context association. Moreover, the magnitude of hippocampal activation correlated with the number, and not the kind, of associated details successfully bound, providing strong evidence for a role of the hippocampus in domain-general associative encoding. By contrast, PrC encoding activation correlated with both nonassociative item encoding as well as associative item-color binding, but not with item-context binding. This pattern suggests that the PrC contributions to memory encoding may be domain-specific and limited to the binding of items with presented item-related features. Critically, together with a separately conducted behavioral study, these data raise the possibility that PrC encoding operations -- in conjunction with hippocampal mechanisms -- contribute to later recollection of presented item details.
Figures
Figure 1
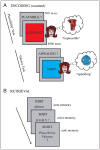
Experimental design. (A) Encoding: Example trials from each of the two scanned encoding tasks (plausibility, valence). In both tasks, subjects were instructed to vividly imagine the referent of the noun in the color presented and to decide either whether this combination was plausible (plausibility task), or whether it was appealing (valence task). If subjects could not imagine the referent of the noun in the given color, a separate button was pressed and those trials were excluded from all analyses. (B) Three-step surprise recognition memory test (unscanned and self-paced), consisting of the assessment of item memory (old/new judgment), associated item-related detail (color memory), and associated item–context detail (task memory). Note that the task memory test was not contingent on the response on the color memory test and vice versa. Question mark responses were allowed to avoid forced-choice guesses.
Figure 2
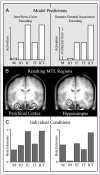
Item/item–color encoding and domain-general associative encoding in the MTL. (A) Parametric model predictions for activation in regions supporting item/item–color (left) and domain-general associative (right) encoding. (B) MTL clusters resulting from the parametric analyses after small volume correction (p < .05). Left: The bilateral perirhinal cortex (y = −6), with the pattern of encoding activation corresponding to item/item–color encoding. Right: The left hippocampus (y = −6), with the pattern of encoding activation corresponding to domain-general associative encoding. Clusters are shown on the mean anatomical image across subjects. (C) Encoding activation (beta estimates) derived from a separate analysis that modeled each condition individually, shown for the perirhinal cortex (left; averaged across left and right hemisphere clusters) and the hippocampus (right). Activation for the individual subsequent memory conditions shows a strong match with the model predictions. M = misses (no item memory); IO = item only (item memory, no color or task memory); IC = item and color (item memory, color memory, no task memory); IT = item and task (item memory, task memory, no color memory); ICT = item and color and task (item memory, color memory, task memory).
Figure 3
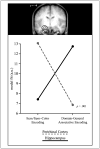
Double dissociation between the contributions of the perirhinal cortex and the hippocampus to episodic encoding. The correspondence (expressed in parameter estimates for the model fits) of encoding activation in the perirhinal cortex (PrC) and the hippocampus is shown for the item/item–color and the domain-general associative model. Activation in the PrC (red; averaged across left and right hemisphere clusters) shows a high fit with the item/item–color model and a low fit with the domain-general associative model, whereas the hippocampus (blue) shows the opposite pattern. The interaction is significant with p = .001.
Figure 4
Division of labor between the perirhinal cortex and the hippocampus for different aspects in memory encoding. Encoding activation (beta estimates) is shown for the perirhinal cortex (red; averaged across left and right hemisphere clusters) and the hippocampus (blue) for critical subsequent memory conditions. (A) Interaction for the difference between M and IO, critical for establishing a role in nonassociative item encoding. (B) Main effect for the difference between IO and IC, with both regions showing enhanced activation for successful item–color associative encoding. (C) Interaction for the difference between IC and ICT, critical for establishing a role in domain-general associative encoding. Stars indicate statistical significance at p < .05. M = misses (no item memory); IO = item only (item memory, no color or task memory); IC = item and color (item memory, color memory, no task memory); ICT = item and color and task (item memory, color memory, task memory).
Similar articles
- Neural correlates of encoding within- and across-domain inter-item associations.
Park H, Rugg MD. Park H, et al. J Cogn Neurosci. 2011 Sep;23(9):2533-43. doi: 10.1162/jocn.2011.21611. Epub 2011 Jan 21. J Cogn Neurosci. 2011. PMID: 21254802 Free PMC article. - Neural activity in the hippocampus and perirhinal cortex during encoding is associated with the durability of episodic memory.
Carr VA, Viskontas IV, Engel SA, Knowlton BJ. Carr VA, et al. J Cogn Neurosci. 2010 Nov;22(11):2652-62. doi: 10.1162/jocn.2009.21381. J Cogn Neurosci. 2010. PMID: 19925190 - Visual integration of objects and scenes increases recollection-based responding despite differential MTL recruitment in young and older adults.
Memel M, Ryan L. Memel M, et al. Hippocampus. 2018 Dec;28(12):886-899. doi: 10.1002/hipo.23011. Epub 2018 Nov 8. Hippocampus. 2018. PMID: 29999561 - Item, context and relational episodic encoding in humans.
Davachi L. Davachi L. Curr Opin Neurobiol. 2006 Dec;16(6):693-700. doi: 10.1016/j.conb.2006.10.012. Epub 2006 Nov 9. Curr Opin Neurobiol. 2006. PMID: 17097284 Review.
Cited by
- The interaction of relational encoding and unitization: Effects on medial temporal lobe processing during retrieval.
Tu HW, Diana RA. Tu HW, et al. Behav Brain Res. 2021 Jan 1;396:112878. doi: 10.1016/j.bbr.2020.112878. Epub 2020 Sep 2. Behav Brain Res. 2021. PMID: 32890598 Free PMC article. - A role for perirhinal cortex in memory for novel object-context associations.
Watson HC, Wilding EL, Graham KS. Watson HC, et al. J Neurosci. 2012 Mar 28;32(13):4473-81. doi: 10.1523/JNEUROSCI.5751-11.2012. J Neurosci. 2012. PMID: 22457495 Free PMC article. - Associative memory for conceptually unitized word pairs in mild cognitive impairment is related to the volume of the perirhinal cortex.
Delhaye E, Mechanic-Hamilton D, Saad L, Das SR, Wisse LEM, Yushkevich PA, Wolk DA, Bastin C. Delhaye E, et al. Hippocampus. 2019 Jul;29(7):630-638. doi: 10.1002/hipo.23063. Epub 2018 Dec 26. Hippocampus. 2019. PMID: 30588714 Free PMC article. - Membrane Resonance in Pyramidal and GABAergic Neurons of the Mouse Perirhinal Cortex.
Binini N, Talpo F, Spaiardi P, Maniezzi C, Pedrazzoli M, Raffin F, Mattiello N, Castagno AN, Masetto S, Yanagawa Y, Dickson CT, Ramat S, Toselli M, Biella GR. Binini N, et al. Front Cell Neurosci. 2021 Jul 22;15:703407. doi: 10.3389/fncel.2021.703407. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34366789 Free PMC article. - Dimensions and mechanisms of memory organization.
de Sousa AF, Chowdhury A, Silva AJ. de Sousa AF, et al. Neuron. 2021 Sep 1;109(17):2649-2662. doi: 10.1016/j.neuron.2021.06.014. Epub 2021 Jul 8. Neuron. 2021. PMID: 34242564 Free PMC article. Review.
References
- Brown MW, Aggleton JP. Recognition memory: What are the roles of the perirhinal cortex and hippocampus? Nature Reviews Neuroscience. 2001;2:51–61. - PubMed
- Buchel C, Holmes AP, Rees G, Friston KJ. Characterizing stimulus–response functions using nonlinear regressors in parametric fMRI experiments. Neuroimage. 1998;8:140–148. - PubMed
- Buckley MJ, Gaffan D. Perirhinal cortex ablation impairs configural learning and paired-associate learning equally. Neuropsychologia. 1998;36:535–546. - PubMed
- Bussey TJ, Saksida LM. The organization of visual object representations: A connectionist model of effects of lesions in perirhinal cortex. European Journal of Neuroscience. 2002;15:355–364. - PubMed
- Cohen NJ, Eichenbaum HE. Memory, amnesia, and the hippocampal system. Cambridge: MIT Press; 1993.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical