Neuroinflammation mediated by IL-1beta increases susceptibility of dopamine neurons to degeneration in an animal model of Parkinson's disease - PubMed (original) (raw)
Neuroinflammation mediated by IL-1beta increases susceptibility of dopamine neurons to degeneration in an animal model of Parkinson's disease
James B Koprich et al. J Neuroinflammation. 2008.
Abstract
Background: The etiology of Parkinson's disease (PD) remains elusive despite identification of several genetic mutations. It is more likely that multiple factors converge to give rise to PD than any single cause. Here we report that inflammation can trigger degeneration of dopamine (DA) neurons in an animal model of Parkinson's disease.
Methods: We examined the effects of inflammation on the progressive 6-OHDA rat model of Parkinson's disease using immunohistochemistry, multiplex ELISA, and cell counting stereology.
Results: We show that a non-toxic dose of lipopolysaccharide (LPS) induced secretion of cytokines and predisposed DA neurons to be more vulnerable to a subsequent low dose of 6-hydroxydopamine. Alterations in cytokines, prominently an increase in interleukin-1beta (IL-1beta), were identified as being potential mediators of this effect that was associated with activation of microglia. Administration of an interleukin-1 receptor antagonist resulted in significant reductions in tumor necrosis factor-alpha and interferon-gamma and attenuated the augmented loss of DA neurons caused by the LPS-induced sensitization to dopaminergic degeneration.
Conclusion: These data provide insight into the etiology of PD and support a role for inflammation as a risk factor for the development of neurodegenerative disease.
Figures
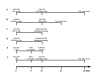
Figure 1
Experimental timelines. Each timeline represents in vivo experimental procedures and postmortem analyses conducted. Number of animals in each group: (A) n = 6, (B) n = 6, (C) n = 7, (D) n = 7, (E) n = 5, (F) n = 8. LPS, lipopolysaccharide; 6-OHDA, 6-hydroxydopamine, SAL, saline; IL-1ra, interluekin-1 receptor antagonist; veh, vehicle; SN, substantia nigra, Str, striatum.
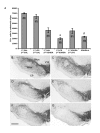
Figure 2
(A) Tyrosine hydroxylase positive (TH+) cell counts in the substantia nigra (SN). (B) Animals received, into the SN, saline (SAL) and 12 days later received an intra-striatal injection of SAL. (C) Animals received lipopolysaccharide (LPS) into the SN and 12 days later received an intra-striatal injection of SAL. (D) Animals received SAL into the SN and 12 days later received an intra-striatal injection of 6-OHDA (5.0 μg). (E) Animals received LPS into the SN and 12 days later received an intra-striatal injection of 6-OHDA (5.0 μg). (F) Animals received an intra-striatal injection of 6-OHDA (5.0 μg) and 12 days later received LPS into the SN. (G) Animals received an intra-striatal injection of 6-OHDA-h (high) (22.5 μg). Letters inside bars of panel A represent respective images in panels below. 21 days was allowed following 6-OHDA injection in all conditions. There was an overall main effect across groups (ANOVA, p < 0.001). LPS injection into the SN was non-toxic to TH+ neurons (B vs. C, NS). LPS injection prior to 6-OHDA administration increased the amount of TH+ cell loss compared to SAL prior to 6-OHDA (D vs. E, *p < 0.05, post-hoc Holm-Sidak). Intra-striatal injection of 6-OHDA followed by injection of LPS in the SN produced no greater cell loss (D vs. F, NS). The high dose of 6-OHDA produced cell loss that was not significantly different from a low dose of 6-OHDA with prior exposure to LPS (E vs. G, NS), however the high dose of 6-OHDA was significantly greater than other conditions receiving the lower dose of 6-OHDA (G vs. D and F, # p < 0.05, post-hoc Holm-Sidak). 1st, first injection; 2nd, second injection; SNpc, pars compacta; SNpr, pars reticulata; VTA, ventral tegmental area; CP, cerebral peduncle; scale bar, 1.0 mm; magnification, 2.5×. Error bars, ± SEM; n = 6 per condition.
Figure 3
Representative coronal sections of microglia in the ventral midbrain at the level of the substantia nigra (SN) visualized by CD11b-immunoreactivity (-ir). (A) activated microglia in the SN of a rat injected in the SN with LPS 12 days prior and (B) the contralateral side. (C) low level of microglia activation 12 days following an intra-striatal injection of 6-OHDA (5.0 μg) and (D) the contralateral side showing resting microglia. Insets are 20× images taken from the area outlined in lower magnification pictures. Scale bar of inset, 100 μm; scale bar of low magnification images (2.5×), 1.0 mm).
Figure 4
Interleukin-1 receptor antagonist (IL-1ra) reduces the amount of tyrosine hydroxylase-immunoreactive (TH-ir) cell loss associated with LPS and 6-OHDA (t-test, *p < 0.05). Each animal received LPS into the substantia nigra (SN), 9 days later started on either IL-1ra or vehicle (Veh) which continued through the experiment, and all animals then received an intra-striatal injection of 6-OHDA (5.0 μg) and were allowed 21 days until post-mortem analyses. Magnification, 2.5×; scale bar, 1.0 mm. Error bars, ± SEM; n = 8 per condition.
Similar articles
- Inflammatory priming of the substantia nigra influences the impact of later paraquat exposure: Neuroimmune sensitization of neurodegeneration.
Mangano EN, Hayley S. Mangano EN, et al. Neurobiol Aging. 2009 Sep;30(9):1361-78. doi: 10.1016/j.neurobiolaging.2007.11.020. Epub 2008 Jan 10. Neurobiol Aging. 2009. PMID: 18187236 - Exogenous corticosterone reduces L-DOPA-induced dyskinesia in the hemi-parkinsonian rat: role for interleukin-1beta.
Barnum CJ, Eskow KL, Dupre K, Blandino P Jr, Deak T, Bishop C. Barnum CJ, et al. Neuroscience. 2008 Sep 22;156(1):30-41. doi: 10.1016/j.neuroscience.2008.07.016. Epub 2008 Jul 12. Neuroscience. 2008. PMID: 18687386 Free PMC article. - Blocking soluble tumor necrosis factor signaling with dominant-negative tumor necrosis factor inhibitor attenuates loss of dopaminergic neurons in models of Parkinson's disease.
McCoy MK, Martinez TN, Ruhn KA, Szymkowski DE, Smith CG, Botterman BR, Tansey KE, Tansey MG. McCoy MK, et al. J Neurosci. 2006 Sep 13;26(37):9365-75. doi: 10.1523/JNEUROSCI.1504-06.2006. J Neurosci. 2006. PMID: 16971520 Free PMC article. - MPTP and 6-hydroxydopamine-induced neurodegeneration as models for Parkinson's disease: neuroprotective strategies.
Grünblatt E, Mandel S, Youdim MB. Grünblatt E, et al. J Neurol. 2000 Apr;247 Suppl 2:II95-102. doi: 10.1007/pl00022909. J Neurol. 2000. PMID: 10991672 Review. - Modeling Parkinson's disease in rats: an evaluation of 6-OHDA lesions of the nigrostriatal pathway.
Deumens R, Blokland A, Prickaerts J. Deumens R, et al. Exp Neurol. 2002 Jun;175(2):303-17. doi: 10.1006/exnr.2002.7891. Exp Neurol. 2002. PMID: 12061862 Review.
Cited by
- MicroRNA-7 targets Nod-like receptor protein 3 inflammasome to modulate neuroinflammation in the pathogenesis of Parkinson's disease.
Zhou Y, Lu M, Du RH, Qiao C, Jiang CY, Zhang KZ, Ding JH, Hu G. Zhou Y, et al. Mol Neurodegener. 2016 Apr 16;11:28. doi: 10.1186/s13024-016-0094-3. Mol Neurodegener. 2016. PMID: 27084336 Free PMC article. - Anti-inflammatory effects of BHBA in both in vivo and in vitro Parkinson's disease models are mediated by GPR109A-dependent mechanisms.
Fu SP, Wang JF, Xue WJ, Liu HM, Liu BR, Zeng YL, Li SN, Huang BX, Lv QK, Wang W, Liu JX. Fu SP, et al. J Neuroinflammation. 2015 Jan 17;12:9. doi: 10.1186/s12974-014-0230-3. J Neuroinflammation. 2015. PMID: 25595674 Free PMC article. - Brain angiotensin and dopaminergic degeneration: relevance to Parkinson's disease.
Labandeira-Garcia JL, Rodriguez-Pallares J, Rodríguez-Perez AI, Garrido-Gil P, Villar-Cheda B, Valenzuela R, Guerra MJ. Labandeira-Garcia JL, et al. Am J Neurodegener Dis. 2012;1(3):226-44. Epub 2012 Nov 18. Am J Neurodegener Dis. 2012. PMID: 23383395 Free PMC article. - Interleukin-1 receptor antagonist reduces neonatal lipopolysaccharide-induced long-lasting neurobehavioral deficits and dopaminergic neuronal injury in adult rats.
Pang Y, Tien LT, Zhu H, Shen J, Wright CF, Jones TK, Mamoon SA, Bhatt AJ, Cai Z, Fan LW. Pang Y, et al. Int J Mol Sci. 2015 Apr 17;16(4):8635-54. doi: 10.3390/ijms16048635. Int J Mol Sci. 2015. PMID: 25898410 Free PMC article. - L-type Cav1.2 calcium channel is involved in 6-hydroxydopamine-induced neurotoxicity in rats.
Wang R, Ma Z, Wang J, Xie J. Wang R, et al. Neurotox Res. 2012 Apr;21(3):266-70. doi: 10.1007/s12640-011-9271-x. Epub 2011 Sep 7. Neurotox Res. 2012. PMID: 21901331
References
- Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, van Dongen JW, Vanacore N, van Swieten JC, Brice A, Meco G, van Duijn CM, Oostra BA, Heutink P. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. doi: 10.1126/science.1077209. - DOI - PubMed
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, DiIorio G, Golbe LI, Nussbaum RL. Mutation in the a-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources