Mechanical stimulation by osmotic and hydrostatic pressure activates Drosophila oocytes in vitro in a calcium-dependent manner - PubMed (original) (raw)
Mechanical stimulation by osmotic and hydrostatic pressure activates Drosophila oocytes in vitro in a calcium-dependent manner
Vanessa L Horner et al. Dev Biol. 2008.
Abstract
Embryogenesis in vertebrates and marine invertebrates begins when a mature oocyte is fertilized, resulting in a rise in intracellular calcium (Ca(2+)) that activates development. Insect eggs activate without fertilization via an unknown signal imparted to the egg during ovulation or egg laying. One hypothesis for the activating signal is that deformation of eggs as they pass through a tight orifice provides a mechanical stimulus to trigger activation. Ovulation could produce two forms of mechanical stimulus: external pressure resulting from the passage of oocytes from the ovary into the narrow oviducts, and osmotic pressure caused by hydration-induced swelling of the oocyte within the oviducts. Ovulation could also trigger activation by placing the oocyte in a new environment that contains an activating substance, such as a particular ion. Here, we provide the first evidence that Drosophila oocytes require Ca(2+) for activation, and that activation can be triggered in vitro by mechanical stimuli, specifically osmotic and hydrostatic pressure. Our results suggest that activation in Drosophila is triggered by a mechanosensitive process that allows external Ca(2+) to enter the oocyte and drive the events of activation. This will allow exploitation of Drosophila genetics to dissect molecular pathways involving Ca(2+) and the activation of development.
Figures
Figure 1
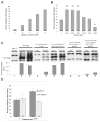
Hydrostatic pressure accelerates VM hardening and protein translation in a Ca2+-dependent manner. (A) VM hardening increases gradually during in vitro activation. (B) Hydrostatic pressure significantly affects VM hardening (Logistic regression, _χ_27= 72.5, P<0.0001). **_P_≤ 0.001, *_P_≤ 0.01 in post-hoc Chi-square analysis between 0 psi and 4000, 6000, 8000, and 14,000 psi (_χ_21= 53.0, 37.6, 45.0, and 8.5, respectively). (C) Hydrostatic pressure accelerates SMG translation in a Ca2+-dependent manner. Graph corresponds to lanes shown directly above; values from scans were background adjusted, normalized to tubulin and averaged over two independent experiments. (D) Hydrostatic pressure increases VM hardening only when external Ca2+ is added to the buffers (compare dark bars to light bars). In panels (A), (B) and (D) the numbers in bars indicate the number of eggs used in each experiment.
Figure 2
Hypo-osmotic swelling generates a mechanical response that requires external Ca2+. (A) GdCl3, (B) BAPTA, and (C) EGTA significantly decrease VM hardening. The numbers in bars indicate the number of eggs used in each experiment. (D) GdCl3, BAPTA, and EGTA reduce SMG translation. Lines between lanes indicate separate experiments. Graph corresponds to lanes shown directly above; values from scans were background adjusted, normalized to tubulin and averaged over two experiments. Hatch bars indicate values greater than graph range (0.77 fertilized and 0.78 unfertilized).
Figure 3
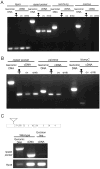
Expression of putative SA ion channels in Drosphila oocytes and early embryos. (A) ripped pocket mRNA is expressed in whole females, ovaries, and 0–2 hour embryos. nanchung and inactive mRNAs are not expressed in ovaries or 0–2 hour embryos; however, expression was not detected in whole females, so it is possible that this method is not sensitive to very low-level expression. Rp49 (Ribosomal protein 49) served as an internal control for genomic DNA and cDNA preparation. (B) painless mRNA is expressed in whole females and ovaries, but not 0–2 hour embryos. NompC mRNA is not expressed in ovaries or 0–2 hour embryos. The internal control in this experiment was ripped pocket. (C) (Top) Line EY12268 contains a P element insertion (triangle) 31 nucleotides upstream of the 5’UTR of rpk. Exons 1–4 of rpk are numbered. (Bottom) In one imprecise excision line, the expression of rpk in ovaries is lowered compared to WT ovaries.
Similar articles
- Calcium waves occur as Drosophila oocytes activate.
Kaneuchi T, Sartain CV, Takeo S, Horner VL, Buehner NA, Aigaki T, Wolfner MF. Kaneuchi T, et al. Proc Natl Acad Sci U S A. 2015 Jan 20;112(3):791-6. doi: 10.1073/pnas.1420589112. Epub 2015 Jan 6. Proc Natl Acad Sci U S A. 2015. PMID: 25564670 Free PMC article. - Ovulation triggers activation of Drosophila oocytes.
Heifetz Y, Yu J, Wolfner MF. Heifetz Y, et al. Dev Biol. 2001 Jun 15;234(2):416-24. doi: 10.1006/dbio.2001.0246. Dev Biol. 2001. PMID: 11397010 - The Drosophila Trpm channel mediates calcium influx during egg activation.
Hu Q, Wolfner MF. Hu Q, et al. Proc Natl Acad Sci U S A. 2019 Sep 17;116(38):18994-19000. doi: 10.1073/pnas.1906967116. Epub 2019 Aug 19. Proc Natl Acad Sci U S A. 2019. PMID: 31427540 Free PMC article. - Calcium and egg activation in Drosophila.
Sartain CV, Wolfner MF. Sartain CV, et al. Cell Calcium. 2013 Jan;53(1):10-5. doi: 10.1016/j.ceca.2012.11.008. Epub 2012 Dec 5. Cell Calcium. 2013. PMID: 23218670 Free PMC article. Review. - Egg Activation at Fertilization.
Machaty Z, Miller AR, Zhang L. Machaty Z, et al. Adv Exp Med Biol. 2017;953:1-47. doi: 10.1007/978-3-319-46095-6_1. Adv Exp Med Biol. 2017. PMID: 27975269 Review.
Cited by
- The spatial and mechanical challenges of female meiosis.
Evans JP, Robinson DN. Evans JP, et al. Mol Reprod Dev. 2011 Oct-Nov;78(10-11):769-77. doi: 10.1002/mrd.21358. Epub 2011 Jul 19. Mol Reprod Dev. 2011. PMID: 21774026 Free PMC article. Review. - Ca2+ signaling during mammalian fertilization: requirements, players, and adaptations.
Wakai T, Vanderheyden V, Fissore RA. Wakai T, et al. Cold Spring Harb Perspect Biol. 2011 Apr 1;3(4):a006767. doi: 10.1101/cshperspect.a006767. Cold Spring Harb Perspect Biol. 2011. PMID: 21441584 Free PMC article. Review. - Control of PNG kinase, a key regulator of mRNA translation, is coupled to meiosis completion at egg activation.
Hara M, Petrova B, Orr-Weaver TL. Hara M, et al. Elife. 2017 May 30;6:e22219. doi: 10.7554/eLife.22219. Elife. 2017. PMID: 28555567 Free PMC article. - TRPV4 Plays a Role in Matrix Stiffness-Induced Macrophage Polarization.
Dutta B, Goswami R, Rahaman SO. Dutta B, et al. Front Immunol. 2020 Dec 14;11:570195. doi: 10.3389/fimmu.2020.570195. eCollection 2020. Front Immunol. 2020. PMID: 33381111 Free PMC article. - Distinct mechanisms regulating mechanical force-induced Ca²⁺ signals at the plasma membrane and the ER in human MSCs.
Kim TJ, Joo C, Seong J, Vafabakhsh R, Botvinick EL, Berns MW, Palmer AE, Wang N, Ha T, Jakobsson E, Sun J, Wang Y. Kim TJ, et al. Elife. 2015 Feb 10;4:e04876. doi: 10.7554/eLife.04876. Elife. 2015. PMID: 25667984 Free PMC article.
References
- Allis CD, Waring GL, Mahowald AP. Mass isolation of pole cells from Drosophila melanogaster. Dev Biol. 1977;56:372–81. - PubMed
- Bers DM, Patton CW, Nuccitelli R. A practical guide to the preparation of Ca2+ buffers. Methods Cell Biol. 1994;40:3–29. - PubMed
- Caldwell RA, Clemo HF, Baumgarten CM. Using gadolinium to identify stretch-activated channels: technical considerations. Am J Physiol. 1998;275:C619–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM044659-14/GM/NIGMS NIH HHS/United States
- GM44659/GM/NIGMS NIH HHS/United States
- R01 GM044659-15/GM/NIGMS NIH HHS/United States
- R01 GM044659-16A1/GM/NIGMS NIH HHS/United States
- R01 GM044659/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous