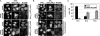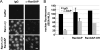The Nup358-RanGAP complex is required for efficient importin alpha/beta-dependent nuclear import - PubMed (original) (raw)
The Nup358-RanGAP complex is required for efficient importin alpha/beta-dependent nuclear import
Saskia Hutten et al. Mol Biol Cell. 2008 May.
Abstract
In vertebrate cells, the nucleoporin Nup358/RanBP2 is a major component of the filaments that emanate from the nuclear pore complex into the cytoplasm. Nup358 forms a complex with SUMOylated RanGAP1, the GTPase activating protein for Ran. RanGAP1 plays a pivotal role in the establishment of a RanGTP gradient across the nuclear envelope and, hence, in the majority of nucleocytoplasmic transport pathways. Here, we investigate the roles of the Nup358-RanGAP1 complex and of soluble RanGAP1 in nuclear protein transport, combining in vivo and in vitro approaches. Depletion of Nup358 by RNA interference led to a clear reduction of importin alpha/beta-dependent nuclear import of various reporter proteins. In vitro, transport could be partially restored by the addition of importin beta, RanBP1, and/or RanGAP1 to the transport reaction. In intact Nup358-depleted cells, overexpression of importin beta strongly stimulated nuclear import, demonstrating that the transport receptor is the most rate-limiting factor at reduced Nup358-concentrations. As an alternative approach, we used antibody-inhibition experiments. Antibodies against RanGAP1 inhibited the enzymatic activity of soluble and nuclear pore-associated RanGAP1, as well as nuclear import and export. Although export could be fully restored by soluble RanGAP, import was only partially rescued. Together, these data suggest a dual function of the Nup358-RanGAP1 complex as a coordinator of importin beta recycling and reformation of novel import complexes.
Figures
Figure 1.
Nup358 promotes importin α/β-dependent nuclear import. (A) Mock-treated or Nup358-depleted cells were transiently transfected with constructs coding for Rev (aa 48-116)-GFP2-cNLS or NS2P (aa 76-97)-GFP2-cNLS, as indicated. Reporter proteins with shorter NES-fragments of the HIV-1-Rev protein showed the same effects (cf. Figure 7, B and C). (B and C) Mock-treated or Nup358-depleted HeLa cells were transfected with a plasmid coding for GR2-GFP2-cNLS and either fixed before (− dexamethasone) or after induction of import by the addition of dexamethasone for 15 min (+ dexamethasone). (A and B) Cells were stained for Nup358 and analyzed by fluorescence microscopy. Bar, 10 μm. (C) The mean distribution of GR2-GFP2-cNLS fluorescence 15 min after induction of import with dexamethasone is shown for mock-treated or Nup358-depleted cells in the three categories: N > C, N = C, and N < C. Bars, SD from the mean of three independent experiments.
Figure 2.
Anti-RanGAP antibodies inhibit nuclear import of BSA-NLS and export of GFP-NFAT in vitro. (A) Anti-RanGAP antibodies inhibit RanGAP activity in permeabilized cells. Digitonin-permeabilized cells were used as a source of RanGAP activity. Cells were preincubated with increasing concentrations of anti-RanGAP antibodies. (B–D) Nuclear import of Cy5-BSA-NLS (B) and export of GFP-NFAT (C and D) were analyzed by fluorescence microscopy (C) or flow cytometry (B and D). Permeabilized GFP-NFAT cells were preincubated with increasing concentrations (B and D) or 300 μg/ml (C) of IgG or anti-RanGAP antibodies, followed by a washing step. (B and D) Reactions contained 2.4 μM Ran, 750 nM importin α, 250 nM importin β, and 225 nM CRM1. Note that nuclear export in D was analyzed in parallel in the very same reactions as nuclear import in B. (C) Reactions contained 2 μM Ran and 250 nM CRM1. Reactions were performed at 4 or 30°C, as indicated. Bar, 20 μm.
Figure 3.
RanGAP reverses the inhibition of nuclear export by anti-RanGAP antibodies. Nuclear export of GFP-NFAT was analyzed by flow cytometry. Permeabilized cells were preincubated with ∼280 μg/ml unspecific IgG or anti-RanGAP antibodies. After a washing step, cells were subjected to export reactions. (A) Reactions contained 5 mg/ml cytosol as a source of transport factors. (B and C) Reactions contained 3.6 μM Ran, 700 nM CRM1, and 350 nM RanGAP (B) or increasing concentrations of RanGAP (C), as indicated. (D) Reactions contained 2.4 μM Ran, 750 nM importin α, 250 nM importin β, and 225 nM CRM1. RanGAP or RanGAP-KR at 150 nM was added to the reactions, as indicated. Reactions were performed at 4 or 30°C, and nuclear export of GFP-NFAT was analyzed by flow cytometry.
Figure 4.
Soluble RanGAP promotes nuclear import in vitro. (A) HeLa cells grown on coverslips were permeabilized, preincubated with IgG or anti-RanGAP, and subjected to nuclear import reactions in the presence of buffer or the transport factors importin α (750 nM), importin β (250 nM), Ran (6 μM), and RanGAP (20 nM), as indicated. Cy3-labeled BSA-NLS was used as import substrate. Bar, 10 μm. (B) Nuclear import was analyzed by flow cytometry, using GFP-NFAT cells. Permeabilized cells were preincubated with increasing concentrations of IgG or anti-RanGAP antibodies, followed by a washing step. Cy5-labeled BSA-NLS was used as import substrate. All reactions contained 2.4 μM Ran, 750 nM importin α, 250 nM importin β, and 225 nM CRM1. RanGAP at 500 nM or RanGAP-KR at 150 nM, was added to the reactions, as indicated. Reactions were performed at 4 or 30°C, as indicated. Bars, variation from the mean of three independent experiments.
Figure 5.
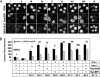
Depletion of Nup358-RanGAP inhibits nuclear import in vitro. (A) Mock-treated and Nup358-depleted cells were mixed, permeabilized, and incubated with FITC-BSA-NLS, 1 μM Ran, and 500 nM importin α in the presence of 100 nM importin β, 25 nM RanBP1, and 200 nM RanGAP, as indicated by the Roman numerals (I–IX; compare conditions in BI. (B) Quantification of nuclear import. Nuclear fluorescence in the presence of Ran (second row) was subtracted as background and import efficiency (IE) was expressed as the percentage of the obtained value in Nup358-depleted cells compared with control cells (bottom line). Error bars, SD from the mean of three independent experiments (50–100 cells each).
Figure 6.
Depletion of Nup358-RanGAP by RNAi. (A) Mock-treated and Nup358-depleted cells were mixed and either fixed before (intact) or after (permeabilized) permeabilization with digitonin. To mimic transport conditions, cells were incubated at 30°C with Ran and an ATP-regenerating system for 30 min. Cells were stained for DNA, Nup358, RanGAP, and importin β, as indicated. Bar, 10 μm. (B) Mock-treated and Nup358-depleted cells were lysed in SDS-sample buffer (t; lanes 1 and 5) or permeabilized and incubated with Ran and an ATP-regenerating system at 30°C. S1 (lanes 2 and 6) corresponds to the supernatant after permeabilization, P (lanes 3 and 7) and S2 (lanes 4 and 8) to the nuclear fraction and the supernatant after the incubation at 30°C, respectively. (C) Mock-treated cells and Nup358-depleted cells were lysed in SDS-sample buffer and analyzed by Western blotting for levels of Nup358 and sumoylated and unsumoylated RanGAP. The equivalents of ∼2 × 105 (100%) or 1 × 105 (50%) cells were loaded. (B and C) α-tubulin served as a loading control.
Figure 7.
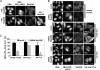
Importin β is the rate-limiting import factor in Nup358-depleted cells. (A) HeLa cells were cotransfected with constructs coding for HIV-1-Rev (aa 48-116)-GFP2-cNLS and HA-Nup358 (aa 2595-2881) at a ratio of 1:7, stained for RanGAP and HA-Nup358, and analyzed by fluorescence microscopy. (B) Mock- or siRNA-treated cells were cotransfected with Rev (aa 68-90)-GFP2-cNLS and either empty vector (top panel), a plasmid coding for HA-importin β (middle panel) or HA-transportin (bottom panel) at a ratio of 1:5, stained for Nup358 and HA-tagged protein, and analyzed by fluorescence microscopy. Bars, 10 μm. A quantification of cells showing a clear nuclear localization (N > C) of the reporter protein in the absence or presence of HA-importin β (HA-Imp β) or HA-transportin (HA-Trn) is shown in C. Error bars, the variation from the mean of two independent experiments (>100 cells each).
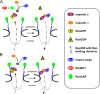
Figure 8.
The model depicts two alternative pathways for importin α/β-dependent nuclear import. (A) Disassembly of recycling importin β bound to RanGTP and assembly of a new import complex occurs in the cytoplasm, promoted by soluble RanGAP and RanBP1. These soluble proteins may also be used for free RanGTP that escapes from the nucleus in an uncomplexed form. (B) Recycling importin β-RanGTP interacts with the Ran-binding domains of Nup358, which, together with associated RanGAP, coordinates GTP-hydrolysis on Ran and formation of a novel import complex.
Similar articles
- The nucleoporin Nup358/RanBP2 promotes nuclear import in a cargo- and transport receptor-specific manner.
Wälde S, Thakar K, Hutten S, Spillner C, Nath A, Rothbauer U, Wiemann S, Kehlenbach RH. Wälde S, et al. Traffic. 2012 Feb;13(2):218-33. doi: 10.1111/j.1600-0854.2011.01302.x. Epub 2011 Nov 21. Traffic. 2012. PMID: 21995724 - Molecular Characterization and Functional Analysis of Annulate Lamellae Pore Complexes in Nuclear Transport in Mammalian Cells.
Raghunayakula S, Subramonian D, Dasso M, Kumar R, Zhang XD. Raghunayakula S, et al. PLoS One. 2015 Dec 7;10(12):e0144508. doi: 10.1371/journal.pone.0144508. eCollection 2015. PLoS One. 2015. PMID: 26642330 Free PMC article. - Ran-dependent docking of importin-beta to RanBP2/Nup358 filaments is essential for protein import and cell viability.
Hamada M, Haeger A, Jeganathan KB, van Ree JH, Malureanu L, Wälde S, Joseph J, Kehlenbach RH, van Deursen JM. Hamada M, et al. J Cell Biol. 2011 Aug 22;194(4):597-612. doi: 10.1083/jcb.201102018. J Cell Biol. 2011. PMID: 21859863 Free PMC article. - Classical nuclear localization signals: definition, function, and interaction with importin alpha.
Lange A, Mills RE, Lange CJ, Stewart M, Devine SE, Corbett AH. Lange A, et al. J Biol Chem. 2007 Feb 23;282(8):5101-5. doi: 10.1074/jbc.R600026200. Epub 2006 Dec 14. J Biol Chem. 2007. PMID: 17170104 Free PMC article. Review. - Importin α: functions as a nuclear transport factor and beyond.
Oka M, Yoneda Y. Oka M, et al. Proc Jpn Acad Ser B Phys Biol Sci. 2018;94(7):259-274. doi: 10.2183/pjab.94.018. Proc Jpn Acad Ser B Phys Biol Sci. 2018. PMID: 30078827 Free PMC article. Review.
Cited by
- Specific armadillo repeat sequences facilitate β-catenin nuclear transport in live cells via direct binding to nucleoporins Nup62, Nup153, and RanBP2/Nup358.
Sharma M, Jamieson C, Johnson M, Molloy MP, Henderson BR. Sharma M, et al. J Biol Chem. 2012 Jan 6;287(2):819-31. doi: 10.1074/jbc.M111.299099. Epub 2011 Nov 21. J Biol Chem. 2012. PMID: 22110128 Free PMC article. Retracted. - Orchestrating Lymphocyte Polarity in Cognate Immune Cell-Cell Interactions.
Bustos-Morán E, Blas-Rus N, Martín-Cófreces NB, Sánchez-Madrid F. Bustos-Morán E, et al. Int Rev Cell Mol Biol. 2016;327:195-261. doi: 10.1016/bs.ircmb.2016.06.004. Epub 2016 Jul 30. Int Rev Cell Mol Biol. 2016. PMID: 27692176 Free PMC article. Review. - YTHDF1 Aggravates the Progression of Cervical Cancer Through m6A-Mediated Up-Regulation of RANBP2.
Wang H, Luo Q, Kang J, Wei Q, Yang Y, Yang D, Liu X, Liu T, Yi P. Wang H, et al. Front Oncol. 2021 Mar 19;11:650383. doi: 10.3389/fonc.2021.650383. eCollection 2021. Front Oncol. 2021. PMID: 33816306 Free PMC article. - Human CRM1 augments production of infectious human and feline immunodeficiency viruses from murine cells.
Elinav H, Wu Y, Coskun A, Hryckiewicz K, Kemler I, Hu Y, Rogers H, Hao B, Ben Mamoun C, Poeschla E, Sutton R. Elinav H, et al. J Virol. 2012 Nov;86(22):12053-68. doi: 10.1128/JVI.01970-12. Epub 2012 Aug 29. J Virol. 2012. PMID: 22933280 Free PMC article. - Herpes simplex virus replication: roles of viral proteins and nucleoporins in capsid-nucleus attachment.
Copeland AM, Newcomb WW, Brown JC. Copeland AM, et al. J Virol. 2009 Feb;83(4):1660-8. doi: 10.1128/JVI.01139-08. Epub 2008 Dec 10. J Virol. 2009. PMID: 19073727 Free PMC article.
References
- Allen N. P., Huang L., Burlingame A., Rexach M. Proteomic analysis of nucleoporin interacting proteins. J. Biol. Chem. 2001;276:29268–29274. - PubMed
- Baake M., Bauerle M., Doenecke D., Albig W. Core histones and linker histones are imported into the nucleus by different pathways. Eur. J. Cell Biol. 2001;80:669–677. - PubMed
- Bischoff F. R., Görlich D. RanBP1 is crucial for the release of RanGTP from importin beta-related nuclear transport factors. FEBS Lett. 1997;419:249–254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous