Persistent alterations in cognitive function and prefrontal dopamine D2 receptors following extended, but not limited, access to self-administered cocaine - PubMed (original) (raw)
Persistent alterations in cognitive function and prefrontal dopamine D2 receptors following extended, but not limited, access to self-administered cocaine
Lisa A Briand et al. Neuropsychopharmacology. 2008 Nov.
Abstract
Drug addicts have deficits in frontocortical function and cognition even long after the discontinuation of drug use. It is not clear, however, whether the cognitive deficits are a consequence of drug use, or are present prior to drug use, and thus are a potential predisposing factor for addiction. To determine if self-administration of cocaine is capable of producing long-lasting alterations in cognition, rats were allowed access to cocaine for either 1 h/day (short access, ShA) or 6 h/day (long access, LgA) for 3 weeks. Between 1 and 30 days after the last self-administration session, we examined performance on a cognitively demanding test of sustained attention that requires an intact medial prefrontal cortex. The expression levels of dopamine D1 and D2 receptor mRNA and D2 protein in the prefrontal cortex were also examined. Early after discontinuation of drug use, LgA (but not ShA) animals were markedly impaired on the sustained attention task. Although the LgA animals improved over time, they continued to show a persistent pattern of performance deficits indicative of a disruption of cognitive flexibility up to 30 days after the discontinuation of drug use. This was accompanied by a significant decrease in DA D2 (but not D1) mRNA in the medial and orbital prefrontal cortex, and D2 receptor protein in the medial prefrontal cortex of LgA (but not ShA) animals. These findings establish that repeated cocaine use is capable of producing persistent alterations in the prefrontal cortex and in cognitive function, and illustrate the usefulness of extended access self-administration procedures for studying the neurobiology of addiction.
Figures
Figure 1
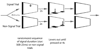
Schematic illustration of the of the sustained attention task. Correct responses on signal trials (hits) and non-signal trials (correct rejections) were rewarded (arrows) and incorrect responses (misses and false alarms, respectively) were not.
Figure 2
A. Mean (+/− SEM) number of cocaine infusions during the first hour of each self-administration session in Experiment I. Both the short (ShA, 1 hr sessions) and the long (LgA, 6 hr sessions) access groups increased their intake over time, but this effect was significantly greater in the LgA group than the ShA group. B. The mean (+/− SEM) number of cocaine infusions over the entire session for LgA and ShA groups.
Figure 3
The effect of extended vs. limited access to self-administered cocaine on performance on the sustained attention task as a function of time following the last self-administration session. The top three panels show the data expressed as a vigilance index (VI), which provides an overall index of performance incorporating both signal and non-signal trials (see Methods). The bottom three panels show the percent correct responses for signal trials (as a function of signal duration; line graph) and non-signal trials (bar graph) separately. The panels on the left (a, d) show performance on the last day of training, prior to any cocaine self-administration experience, in the animals that will eventually be given limited (ShA) or extended (LgA) access to cocaine, or no treatment (No Drug). The groups were balanced to equate performance at the end of training, so there are no group differences on any measure at this point in time. The panels in the middle (b, e) show performance one day following the final self-administration session. Panel b shows that at this time there was a significant decrease in VI in the LgA group relative to both the No Drug and ShA groups, which did not differ from one another. Panel e shows that the LgA group performed more poorly than either the ShA or No Drug groups on both signal and non-signal trials, and the ShA and No Drug groups did not differ from one another on either trial type (* differs from ShA and No Drug groups as determined by Fisher’s Tests). The panels on the right (c, f) show performance when the same animals were retested 14 days following the final self-administration session. Panel c shows that at this time there was still a significant decrease in VI in the LgA group, relative to both the No Drug and ShA groups. However, by this time the deficit was primarily a function of impaired performance on non-signal trials (see Panel f, * differs from ShA and No Drug groups as determined by Fisher’s Tests).
Figure 4
The effect of one week of “retraining” on the sustained attention task beginning 14 days after the last self-administration session. Data are shown as percent correct responses (a) on signal trials in which the signal duration was 500 msec, and (b) on non-signal trials. There were no group differences on signal trials over this period of time. However, on non-signal trials the LgA group showed significantly fewer correct responses than either of the other two groups.
Figure 5
The effect of extended vs. limited access to self-administered cocaine on response latency and omission rate (Mean ± SEM) as a function of time following the last self-administration session in Experiment I. There were no group differences on either measure on either day.
Figure 6
A. Mean (+/− SEM) number of cocaine infusions during the first hour of each self-administration session in Experiment II. The LgA group significantly increased their intake over time, but there were no significant changes in cocaine intake over time in the ShA group. B. The mean (+/− SEM) number of cocaine infusions over the entire session for LgA and ShA groups.
Figure 7
The effect of extended vs. limited access to self-administered cocaine on performance on the sustained attention task as a function of time following the last self-administration session in Experiment 2. The data are presented as described for Figure 3. As in Experiment I, there was a significant decrease in VI in the LgA group relative to both the No Drug and ShA groups when animals were tested one day after the last self-administration session, and the latter two groups did not differ from one another (Panel b). Panel e shows that the LgA group made significantly fewer correct responses on signal trials, but the effect on non-signal trials was not statistically significant, presumably because a relatively small number of animals were tested on this day. The panels on the right (c, f) show the performance of an independent group of animals tested for the first time 30 days following the final self-administration session. At this time there were no significant group differences on VI. Panel f shows that, as in Experiment I, when tested long after the last self-administration session the LgA group was not impaired in their performance on signal trials but was impaired on non-signal trials (* differs from ShA and No Drug groups as determined by Fisher’s Tests).
Figure 8
Dopamine D2 receptor (a) and D1 receptor (b) mRNA expression in the medial prefrontal cortex of rats allowed extended (LgA) or limited (SgA) access to self-administered cocaine, and then examined either 4 or 33 days after the last self-administration session. The data represent the mean (+ SEM) levels of mRNA in the LgA and SgA groups expressed as a percent of the average amount of mRNA in the No Drug control group (represented by the horizontal dashed lines). The LgA group showed a significant decrease in D2 receptor mRNA expression, relative to both the No Drug control group and the ShA group, which did not differ from one another (*, differs from ShA and No Drug groups). There were no group differences in D1 mRNA expression in the medial prefrontal cortex.
Figure 9
Dopamine D2 receptor (a) and D1 receptor (b) mRNA expression in the orbital prefrontal cortex of rats allowed extended (LgA) or limited (ShA) access to self-administered cocaine, and then examined either 4 or 33 days after the last self-administration session. The data represent the mean (+ SEM) levels of mRNA in the LgA and ShA groups expressed as a percent of the average amount of mRNA in the no drug control group (see horizontal dashed lines). The LgA group showed a significant decrease in D2 receptor mRNA expression in the orbitofrontal cortex relative to both the ShA and control animals at both withdrawal time points. There were no significant group differences in D1 receptor mRNA expression.
Figure 10
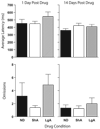
Dopamine D2 receptor protein levels in the medial prefrontal cortex of rats allowed extended (LgA), limited (ShA) or no (ND) access to self-administered cocaine examined either 3 or 30 days after the last self-administration session. The data represent the mean (+SEM) of D2 receptor protein levels relative to an internal control. The LgA group showed a significant decrease in D2 receptor protein levels, relative to both the No Drug control group and the ShA group, which did not differ from one another. As there was a significant effect of group, but no effect of withdrawal time and no group by time interaction, the data were collapsed across the two time points to demonstrate that the group effect is due to a decrease in LgA group (panel b; * differs from ShA and No Drug groups). No group differences were seen in a beta-actin control.
Similar articles
- Changes in levels of D1, D2, or NMDA receptors during withdrawal from brief or extended daily access to IV cocaine.
Ben-Shahar O, Keeley P, Cook M, Brake W, Joyce M, Nyffeler M, Heston R, Ettenberg A. Ben-Shahar O, et al. Brain Res. 2007 Feb 2;1131(1):220-8. doi: 10.1016/j.brainres.2006.10.069. Epub 2006 Dec 11. Brain Res. 2007. PMID: 17161392 Free PMC article. - Cocaine exposure during the early postnatal period diminishes medial frontal cortex Gs coupling to dopamine D1-like receptors in adult rat.
Zhao N, Wang HY, Dow-Edwards D. Zhao N, et al. Neurosci Lett. 2008 Jun 20;438(2):159-62. doi: 10.1016/j.neulet.2008.04.014. Epub 2008 Apr 10. Neurosci Lett. 2008. PMID: 18455307 Free PMC article. - Addiction-related alterations in D1 and D2 dopamine receptor behavioral responses following chronic cocaine self-administration.
Edwards S, Whisler KN, Fuller DC, Orsulak PJ, Self DW. Edwards S, et al. Neuropsychopharmacology. 2007 Feb;32(2):354-66. doi: 10.1038/sj.npp.1301062. Epub 2006 Mar 15. Neuropsychopharmacology. 2007. PMID: 16541082 - Neural substrates of cognitive inflexibility after chronic cocaine exposure.
Stalnaker TA, Takahashi Y, Roesch MR, Schoenbaum G. Stalnaker TA, et al. Neuropharmacology. 2009;56 Suppl 1(Suppl 1):63-72. doi: 10.1016/j.neuropharm.2008.07.019. Epub 2008 Jul 22. Neuropharmacology. 2009. PMID: 18692512 Free PMC article. Review. - Dorsal medial prefrontal cortex (MPFC) circuitry in rodent models of cocaine use: implications for drug addiction therapies.
Jasinska AJ, Chen BT, Bonci A, Stein EA. Jasinska AJ, et al. Addict Biol. 2015 Mar;20(2):215-26. doi: 10.1111/adb.12132. Epub 2014 Mar 13. Addict Biol. 2015. PMID: 24620898 Free PMC article. Review.
Cited by
- Synaptic Cytoskeletal Plasticity in the Prefrontal Cortex Following Psychostimulant Exposure.
DePoy LM, Gourley SL. DePoy LM, et al. Traffic. 2015 Sep;16(9):919-40. doi: 10.1111/tra.12295. Epub 2015 Jun 1. Traffic. 2015. PMID: 25951902 Free PMC article. Review. - Selective activation of Dopamine D3 receptors and norepinephrine transporter blockade enhances sustained attention.
Marshall CA, Brodnik ZD, Mortensen OV, Reith MEA, Shumsky JS, Waterhouse BD, España RA, Kortagere S. Marshall CA, et al. Neuropharmacology. 2019 Apr;148:178-188. doi: 10.1016/j.neuropharm.2019.01.003. Epub 2019 Jan 8. Neuropharmacology. 2019. PMID: 30633928 Free PMC article. - Mechanisms of Shared Vulnerability to Post-traumatic Stress Disorder and Substance Use Disorders.
María-Ríos CE, Morrow JD. María-Ríos CE, et al. Front Behav Neurosci. 2020 Jan 31;14:6. doi: 10.3389/fnbeh.2020.00006. eCollection 2020. Front Behav Neurosci. 2020. PMID: 32082127 Free PMC article. Review. - Repeated amphetamine exposure disrupts dopaminergic modulation of amygdala-prefrontal circuitry and cognitive/emotional functioning.
Tse MT, Cantor A, Floresco SB. Tse MT, et al. J Neurosci. 2011 Aug 3;31(31):11282-94. doi: 10.1523/JNEUROSCI.1810-11.2011. J Neurosci. 2011. PMID: 21813688 Free PMC article. - Diminished role of dopamine D1-receptor signaling with the development of an addicted phenotype in rats.
Ramôa CP, Doyle SE, Lycas MD, Chernau AK, Lynch WJ. Ramôa CP, et al. Biol Psychiatry. 2014 Jul 1;76(1):8-14. doi: 10.1016/j.biopsych.2013.09.028. Epub 2013 Oct 4. Biol Psychiatry. 2014. PMID: 24199666 Free PMC article.
References
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. - PubMed
- Beveridge TJ, Smith HR, Daunais JB, Nader MA, Porrino LJ. Chronic cocaine self-administration is associated with altered functional activity in the temporal lobes of non human primates. Eur J Neurosci. 2006;23:3109–3118. - PubMed
- Bolla KI, Cadet JL, London ED. The neuropsychiatry of chronic cocaine abuse. J Neuropsychiatry Clin Neurosci. 1998;10:280–289. - PubMed
- Bolla KI, Rothman R, Cadet JL. Dose-related neurobehavioral effects of chronic cocaine use. JNeuropsychiatry ClinNeurosci. 1999;11:361–369. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical