Aquaporins--new players in cancer biology - PubMed (original) (raw)
Review
Aquaporins--new players in cancer biology
A S Verkman et al. J Mol Med (Berl). 2008 May.
Abstract
The aquaporins (AQPs) are small, integral-membrane proteins that selectively transport water across cell plasma membranes. A subset of AQPs, the aquaglyceroporins, also transport glycerol. AQPs are strongly expressed in tumor cells of different origins, particularly aggressive tumors. Recent discoveries of AQP involvement in cell migration and proliferation suggest that AQPs play key roles in tumor biology. AQP1 is ubiquitously expressed in tumor vascular endothelium, and AQP1-null mice show defective tumor angiogenesis resulting from impaired endothelial cell migration. AQP-expressing cancer cells show enhanced migration in vitro and greater local tumor invasion, tumor cell extravasation, and metastases in vivo. AQP-dependent cell migration may involve AQP-facilitated water influx into lamellipodia at the front edge of migrating cells. The aquaglyceroporin AQP3, which is found in normal epidermis and becomes upregulated in basal cell carcinoma, facilitates cell proliferation in different cell types. Remarkably, AQP3-null mice are resistant to skin tumorigenesis by a mechanism that may involve reduced tumor cell glycerol metabolism and ATP generation. Together, the data suggest that AQP expression in tumor cells and tumor vessels facilitates tumor growth and spread, suggesting AQP inhibition as a novel antitumor therapy.
Figures
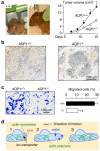
Fig. 1
Impaired angiogenesis and endothelial cell migration in AQP1 knockout mice. a Tumor in wild-type vs AQP1 null mouse, 2 weeks after subcutaneous injection of 106 B16F10 melanoma cells (left). Tumor growth data (10 mice per group, P<0.001) (right). b Tumor tissue stained with the endothelial marker isolectin-B4 (brown). c Migration of aortic endothelial cells for 6 h towards 10% serum shown in a transwell assay (left). Picture shows migrated wild-type and AQP1 null endothelial cells (stained with Coomassie blue) after scraping. Data summary (S.E., _n_=16–20, asterisk P<0.001) (right). d Proposed mechanism of AQP-facilitated endothelial cell migration: 1 Actin depolymerization and ion movements increase osmolality at the anterior end of the cell. 2 Water entry increases local hydrostatic pressure, producing cell membrane expansion to form a protrusion. AQP polarizes to the leading edge of the cell membrane facilitating water entry into the cell. 3 Actin repolymerizes stabilizing the protrusion. Adapted from [14, 17]
Fig. 2
Increased migration and metastasis of AQP-expressing tumor cells. a Control (red fluorescent) and AQP1-expressing (green fluorescent) tumor cells were mixed 1:1 and applied to transwell filters. The upper chamber contained 1% serum and the lower chamber contained 10% serum. Fluorescence micrographs showing red and green cells before (left) and after (middle) scraping nonmigrated cells from the upper surface of the porous membrane (scale bar 50 μm). Summary of ratio of AQP1-expressing vs control (+AQP1/−AQP1) cells at 6 h before and after scraping of nonmigrating cells (asterisk P<0.05) (right). b Hematoxylin and eosinstained paraffin sections of mouse lung tissue at 14 days after tail vein injection of 106 control or AQP1-expressing tumor cells (left). Tumor metastases indicated by arrows. Number of metastases per lung, and area per metastasis (S.E.) (right). Adapted from [16]
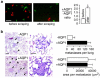
Fig. 3
AQP3 expression in human squamous cell carcinoma and protection against cutaneous papillomas in AQP3-null mice. a AQP3 immunostaining in human skin squamous cell carcinoma. Bar, 50 μm. b Dorsal skin of mice was treated with a single application of DMBA, followed by twice-weekly applications of TPA for 20 weeks (left). Representative photographs showing multiple papillomas in wild-type mouse but no papillomas in AQP3 null mouse. Percentage of mice with papillomas (right). c Proposed cellular mechanism of AQP3-facilitated tumorigenesis. Adapted from [18]
Similar articles
- Aquaporins in sepsis- an update.
Rump K, Adamzik M. Rump K, et al. Front Immunol. 2024 Oct 31;15:1495206. doi: 10.3389/fimmu.2024.1495206. eCollection 2024. Front Immunol. 2024. PMID: 39544938 Free PMC article. Review. - Aquaporins and cell migration.
Papadopoulos MC, Saadoun S, Verkman AS. Papadopoulos MC, et al. Pflugers Arch. 2008 Jul;456(4):693-700. doi: 10.1007/s00424-007-0357-5. Epub 2007 Oct 30. Pflugers Arch. 2008. PMID: 17968585 Free PMC article. Review. - Non-Transport Functions of Aquaporins.
Li X, Yang B. Li X, et al. Adv Exp Med Biol. 2023;1398:65-80. doi: 10.1007/978-981-19-7415-1_5. Adv Exp Med Biol. 2023. PMID: 36717487 - More than just water channels: unexpected cellular roles of aquaporins.
Verkman AS. Verkman AS. J Cell Sci. 2005 Aug 1;118(Pt 15):3225-32. doi: 10.1242/jcs.02519. J Cell Sci. 2005. PMID: 16079275 Review. - Expression and function of aquaporins in human skin: Is aquaporin-3 just a glycerol transporter?
Boury-Jamot M, Sougrat R, Tailhardat M, Le Varlet B, Bonté F, Dumas M, Verbavatz JM. Boury-Jamot M, et al. Biochim Biophys Acta. 2006 Aug;1758(8):1034-42. doi: 10.1016/j.bbamem.2006.06.013. Epub 2006 Jun 18. Biochim Biophys Acta. 2006. PMID: 16872579
Cited by
- Aquaporin Modulation by Cations, a Review.
Mom R, Mocquet V, Auguin D, Réty S. Mom R, et al. Curr Issues Mol Biol. 2024 Jul 24;46(8):7955-7975. doi: 10.3390/cimb46080470. Curr Issues Mol Biol. 2024. PMID: 39194687 Free PMC article. Review. - Aquaporin 1 knockdown inhibits triple-negative breast cancer cell proliferation and invasion in vitro and in vivo.
Ji Y, Liao X, Jiang Y, Wei W, Yang H. Ji Y, et al. Oncol Lett. 2021 Jun;21(6):437. doi: 10.3892/ol.2021.12698. Epub 2021 Apr 1. Oncol Lett. 2021. PMID: 33868475 Free PMC article. - Aquaporins Involvement in Pancreas Physiology and in Pancreatic Diseases.
Arsenijevic T, Perret J, Van Laethem JL, Delporte C. Arsenijevic T, et al. Int J Mol Sci. 2019 Oct 11;20(20):5052. doi: 10.3390/ijms20205052. Int J Mol Sci. 2019. PMID: 31614661 Free PMC article. Review. - Aquaporin 8ab is required in zebrafish embryonic intestine development.
Wang S, Qin Y, Sheng J, Duan X, Shen L, Liu D. Wang S, et al. Acta Biochim Biophys Sin (Shanghai). 2022 Jan 25;54(7):952-960. doi: 10.3724/abbs.2022077. Acta Biochim Biophys Sin (Shanghai). 2022. PMID: 35880566 Free PMC article. - The Water Transport System in Astrocytes-Aquaporins.
Zhou Z, Zhan J, Cai Q, Xu F, Chai R, Lam K, Luan Z, Zhou G, Tsang S, Kipp M, Han W, Zhang R, Yu ACH. Zhou Z, et al. Cells. 2022 Aug 18;11(16):2564. doi: 10.3390/cells11162564. Cells. 2022. PMID: 36010640 Free PMC article. Review.
References
- Verkman AS, Mitra AK. Structure and function of aquaporin water channels. Am J Physiol. 2000;278:F13–F28. - PubMed
- Agre P, Kozono D. Aquaporin water channels: molecular mechanisms for human diseases. FEBS Lett. 2003;555:72–78. - PubMed
- Verkman AS. More than just water channels: unexpected cellular roles of aquaporins. J Cell Sci. 2005;118:3225–3232. - PubMed
- Warth A, Kroger S, Wolburg H. Redistribution of aquaporin-4 in human glioblastoma correlates with loss of agrin immunoreactivity from brain capillary basal laminae. Acta Neuropathol. 2004;107:311–318. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL73856/HL/NHLBI NIH HHS/United States
- R01 EY013574/EY/NEI NIH HHS/United States
- R01 EB000415/EB/NIBIB NIH HHS/United States
- R01 DK035124/DK/NIDDK NIH HHS/United States
- EY13574/EY/NEI NIH HHS/United States
- DK35124/DK/NIDDK NIH HHS/United States
- EB00415/EB/NIBIB NIH HHS/United States
- HL59198/HL/NHLBI NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- R01 HL073856/HL/NHLBI NIH HHS/United States
- P30 DK072517/DK/NIDDK NIH HHS/United States
- DK72517/DK/NIDDK NIH HHS/United States
- R01 HL059198/HL/NHLBI NIH HHS/United States
- R37 DK035124/DK/NIDDK NIH HHS/United States
- R37 EB000415/EB/NIBIB NIH HHS/United States
LinkOut - more resources
Full Text Sources