Concepts in gene therapy for cartilage repair - PubMed (original) (raw)
Review
Concepts in gene therapy for cartilage repair
Andre F Steinert et al. Injury. 2008 Apr.
Abstract
Once articular cartilage is injured, it has a very limited capacity for self repair. Although current surgical therapeutic procedures for cartilage repair are clinically useful, they cannot restore a normal articular surface. Current research offers a growing number of bioactive reagents, including proteins and nucleic acids, that may be used to augment various aspects of the repair process. As these agents are difficult to administer effectively, gene-transfer approaches are being developed to provide their sustained synthesis at sites of repair. To augment regeneration of articular cartilage, therapeutic genes can be delivered to the synovium or directly to the cartilage lesion. Gene delivery to the cells of the synovial lining is generally considered more suitable for chondroprotective approaches, based on the expression of anti-inflammatory mediators. Gene transfer targeted at cartilage defects can be achieved by either direct vector administration to cells located at or surrounding the defects, or by transplantation of genetically modified chondrogenic cells into the defect. Several studies have shown that exogenous cDNAs encoding growth factors can be delivered locally to sites of cartilage damage, where they are expressed at therapeutically relevant levels. Furthermore, data is beginning to emerge indicating that efficient delivery and expression of these genes is capable of influencing a repair response toward the synthesis of a more hyaline cartilage repair tissue in vivo. This review presents the current status of gene therapy for cartilage healing and highlights some of the remaining challenges.
Figures
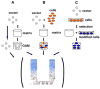
Figure 1
Gene transfer approaches for the treatment of cartilage defects. (A) For in vivo gene transfer, free vector is either injected directly into the joint space, or incorporated into a biologically compatible matrix before implantation into a cartilage defect (gene activated matrix (GAM) implantation). Resident cells that encounter the vector acquire the desired gene, and genetically modified cells secrete the transgene products that influence the regeneration of articular cartilage. (B) Abbreviated ex vivo genetically enhanced tissue engineering to treat cartilage defects. A vector is incorporated into the matrix together with cells that are harvested at the same operative setting, such as stromal cells from bone marrow aspirates. (C) Ex vivo genenetically enhanced tissue engineering for cartilage repair involves the harvest and expansion of target cells in vitro, which are subsequently infected with the desired vector. The transduced cells then may be selected, and seeded into a biological matrix before the construct is transplanted into a cartilage defect. Depending on the approach chosen, ubiquitous or local transgene expression (TGE) is induced by the genetically modified cells, and the gene products could beneficially influence cartilage repair by either transplanted cells as well as those that may migrate into the defect site.
Similar articles
- Development of gene-based therapies for cartilage repair.
Palmer G, Pascher A, Gouze E, Gouze JN, Betz O, Spector M, Robbins PD, Evans CH, Ghivizzani SC. Palmer G, et al. Crit Rev Eukaryot Gene Expr. 2002;12(4):259-73. doi: 10.1615/critreveukaryotgeneexpr.v12.i4.20. Crit Rev Eukaryot Gene Expr. 2002. PMID: 12641395 Review. - Cell-based articular cartilage repair: the link between development and regeneration.
Caldwell KL, Wang J. Caldwell KL, et al. Osteoarthritis Cartilage. 2015 Mar;23(3):351-62. doi: 10.1016/j.joca.2014.11.004. Epub 2014 Nov 11. Osteoarthritis Cartilage. 2015. PMID: 25450846 Free PMC article. Review. - Mesenchymal Stem/Progenitor Cells Derived from Articular Cartilage, Synovial Membrane and Synovial Fluid for Cartilage Regeneration: Current Status and Future Perspectives.
Huang YZ, Xie HQ, Silini A, Parolini O, Zhang Y, Deng L, Huang YC. Huang YZ, et al. Stem Cell Rev Rep. 2017 Oct;13(5):575-586. doi: 10.1007/s12015-017-9753-1. Stem Cell Rev Rep. 2017. PMID: 28721683 Review. - Gene-based approaches for the repair of articular cartilage.
Trippel SB, Ghivizzani SC, Nixon AJ. Trippel SB, et al. Gene Ther. 2004 Feb;11(4):351-9. doi: 10.1038/sj.gt.3302201. Gene Ther. 2004. PMID: 14724680 Review. - Acceleration of cartilage repair by genetically modified chondrocytes over expressing bone morphogenetic protein-7.
Hidaka C, Goodrich LR, Chen CT, Warren RF, Crystal RG, Nixon AJ. Hidaka C, et al. J Orthop Res. 2003 Jul;21(4):573-83. doi: 10.1016/S0736-0266(02)00264-4. J Orthop Res. 2003. PMID: 12798054
Cited by
- Stem cells and gene therapy for cartilage repair.
Longo UG, Petrillo S, Franceschetti E, Berton A, Maffulli N, Denaro V. Longo UG, et al. Stem Cells Int. 2012;2012:168385. doi: 10.1155/2012/168385. Epub 2012 Feb 16. Stem Cells Int. 2012. PMID: 22481959 Free PMC article. - Rejuvenated Stem/Progenitor Cells for Cartilage Repair Using the Pluripotent Stem Cell Technology.
Nakayama N, Ravuri S, Huard J. Nakayama N, et al. Bioengineering (Basel). 2021 Apr 10;8(4):46. doi: 10.3390/bioengineering8040046. Bioengineering (Basel). 2021. PMID: 33920285 Free PMC article. Review. - Functionalized nanostructures with application in regenerative medicine.
Perán M, García MA, López-Ruiz E, Bustamante M, Jiménez G, Madeddu R, Marchal JA. Perán M, et al. Int J Mol Sci. 2012;13(3):3847-3886. doi: 10.3390/ijms13033847. Epub 2012 Mar 22. Int J Mol Sci. 2012. PMID: 22489186 Free PMC article. Review. - Hypertrophy is induced during the in vitro chondrogenic differentiation of human mesenchymal stem cells by bone morphogenetic protein-2 and bone morphogenetic protein-4 gene transfer.
Steinert AF, Proffen B, Kunz M, Hendrich C, Ghivizzani SC, Nöth U, Rethwilm A, Eulert J, Evans CH. Steinert AF, et al. Arthritis Res Ther. 2009;11(5):R148. doi: 10.1186/ar2822. Epub 2009 Oct 2. Arthritis Res Ther. 2009. PMID: 19799789 Free PMC article. - 3D Printed Multiphasic Scaffolds for Osteochondral Repair: Challenges and Opportunities.
Doyle SE, Snow F, Duchi S, O'Connell CD, Onofrillo C, Di Bella C, Pirogova E. Doyle SE, et al. Int J Mol Sci. 2021 Nov 17;22(22):12420. doi: 10.3390/ijms222212420. Int J Mol Sci. 2021. PMID: 34830302 Free PMC article. Review.
References
- Ahrens M, Ankenbauer T, Schroder D, et al. Expression of human bone morphogenetic proteins-2 or -4 in murine mesenchymal progenitor C3H10T1/2 cells induces differentiation into distinct mesenchymal cell lineages. DNA Cell Biol. 1993;12(10):871–880. - PubMed
- Alaaeddine N, Di Battista JA, Pelletier JP, et al. Differential effects of IL-8, LIF (pro-inflammatory) and IL-11 (anti-inflammatory) on TNF-alpha-induced PGE(2)release and on signalling pathways in human OA synovial fibroblasts. Cytokine. 1999;11(12):1020–1030. - PubMed
- Alaaeddine N, Di Battista JA, Pelletier JP, et al. Inhibition of tumor necrosis factor alpha-induced prostaglandin E2 production by the antiinflammatory cytokines interleukin-4, interleukin-10, and interleukin-13 in osteoarthritic synovial fibroblasts: distinct targeting in the signaling pathways. Arthritis Rheum. 1999;42(4):710–718. - PubMed
- Apparailly F, Verwaerde C, Jacquet C, et al. Adenovirus-mediated transfer of viral IL-10 gene inhibits murine collagen-induced arthritis. J Immunol. 1998;160(11):5213–5220. - PubMed
- Apparailly F, Millet V, Noel D, et al. Tetracycline-inducible interleukin-10 gene transfer mediated by an adeno-associated virus: application to experimental arthritis. Hum Gene Ther. 2002;13(10):1179–1188. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials