How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? - PubMed (original) (raw)
Review
How do BCL-2 proteins induce mitochondrial outer membrane permeabilization?
Jerry E Chipuk et al. Trends Cell Biol. 2008 Apr.
Abstract
The mitochondrial pathway of apoptosis proceeds when molecules sequestered between the outer and inner mitochondrial membranes are released to the cytosol by mitochondrial outer membrane permeabilization (MOMP). This process is controlled by the BCL-2 family, which is composed of both pro- and anti-apoptotic proteins. Although there is no disagreement that BCL-2 proteins regulate apoptosis, the mechanism leading to MOMP remains controversial. Current debate focuses on what interactions within the family are crucial to initiate MOMP. Specifically, do the BH3-only proteins directly engage BAX and/or BAK activation or do these proteins solely promote apoptosis by neutralization of anti-apoptotic BCL-2 proteins? We describe these models and contend that BH3-only proteins must perform both functions to efficiently engage MOMP and apoptosis.
Figures
Figure 1
The BCL-2 family of proteins is divided into three functional groups based on their composition of BCL-2 homology domains. The anti-apoptotic members include BCL-2, BCL-xL, BCL-w, A1 and MCL-1 and contain four BCL-2 homology domains (designated BH1–4). The pro-apoptotic multi-domains (BAX, BAK and BOK*) contain BH1–3 domains (*there is little evidence that BOK is a functional effector molecule). The BH3-only proteins are structurally diverse and contain only one conserved region, the BH3 (e.g. leucine-x-x-x-x-aspartic acid, where x is any amino acid). Often, the BH3-only proteins are subdivided into direct activators (e.g. BID an d BIM) and de-repressors/sensitizers (e.g. BAD, BIK, BMF, bNIP3, HRK, Noxa and PUMA). The α helices of each protein are designated and the regions contained within each BH domain are illustrated by bold lines under each protein. The hydrophobic carboxyl terminal transmembrane domain (TM) of each protein is based on in silico predictions and/or structural data and is not necessarily present in each member. Also, a typical BH3 domain might not be absolutely required for every BH3-only protein to induce cell death; for example, deletion of the BH3 domain in bNIP3 does not alter its anti-apoptotic binding or pro-apoptotic activity [52].
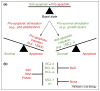
Figure 2
The balance of anti-apoptotic and pro-apoptotic BCL-2 proteins dictates cellular fate. (a) The rheostat model. In a hypothetical basal state, the number of anti-apoptotic and pro-apoptotic molecules is equal; tipping this balance dictates cellular fate. If a stress (e.g. DNA damage) is applied, the induction of pro-apoptotic molecules provides the signal to engage MOMP. On the contrary, growth factor addition would promote cellular survival by increasing the amount of anti-apoptotic proteins. (b) The anti-apoptotic protein neutralization model. The BH3-only proteins BID, BIM and PUMA engage MOMP because they bind and neutralize all anti-apoptotic BCL-2 members. A combination of other BH3-only proteins is required to promote apoptosis because each neutralizes only a subset of anti-apoptotic BCL-2 proteins (e.g. BAD and Noxa neutralize BCL-2/BCL-xL/BCL-w and MCL-1/A1, respectively). This model contends that the BCL-2 effector molecules are sufficiently active to oligomerize and promote MOMP once anti-apoptotic proteins are neutralized.
Figure 3
The direct activation model: mechanisms of pro-apoptotic effector activation by BH3-only proteins. (a) Central to MOMP is the activation and oligomerization of BAX or BAK. These proteins, once activated by a BH3-only protein, create oligomeric proteolipid pores in the outer mitochondrial membrane that permit the release of intermembrane space proteins to the cytosol. (b) Direct activator BH3-only proteins (e.g. BIM and BID) induce the oligomerization and activation of BAX or BAK in the absence of other proteins. Through a transient interaction with BAX or BAK, the direct activator BH3-only proteins (BID is shown in this example), or peptides derived from the BH3 region, induce MOMP and cytochrome c release. This is often referred to as the ‘hit and run’ mechanism for effector activation. (c) A subset of BH3-only proteins, the de-repressors/sensitizers, cannot induce the activation of BAX or BAK alone. In this scenario, a direct activator BH3-only protein is sequestered by an anti-apoptotic BCL-2 protein. Following stress, a de-repressor/sensitizer BH3-only protein is induced, either by transcriptional up-regulation or by post-translat ional modification, and this protein then binds to an anti-apoptotic BCL-2 protein, promoting the release of a sequestered, direct activator BH3-only protein. In this example, B IM istonically sequestered by MCL-1, and the induction of Noxa enables the release of BIM to engage MOMP. If cells constitutively harbor a sequestered direct activator protein, they are referred to as being ‘primed for death’ or ‘BCL-2 addicted’. Not shown in this figure is the potential influence of Noxa-induced MCL-1 degradation after binding, which might have important implications in maintaining anti-apoptotic levels to preserve outer mitochondrial membrane integrity [53]. (d) Cells are sensitized to undergo MOMP when de-repressor/sensitizer BH3-only proteins are constitutively inhibiting anti-apoptotic BCL-2 proteins, and any future induction of BID or BIM cannot be tolerated. This scenario is referred to as ‘sensitized for death’. In this example, BCL-xL is inhibited by BAD, and the induction of BIM engages MOMP; in the absence of B AD expression, the MOMP signal would have been inhibited by BCL-xL.
Similar articles
- MOMP in the absence of BH3-only proteins.
García Sáez AJ, Villunger A. García Sáez AJ, et al. Genes Dev. 2016 Apr 15;30(8):878-80. doi: 10.1101/gad.281519.116. Genes Dev. 2016. PMID: 27083995 Free PMC article. - BH3 domains other than Bim and Bid can directly activate Bax/Bak.
Du H, Wolf J, Schafer B, Moldoveanu T, Chipuk JE, Kuwana T. Du H, et al. J Biol Chem. 2011 Jan 7;286(1):491-501. doi: 10.1074/jbc.M110.167148. Epub 2010 Nov 1. J Biol Chem. 2011. PMID: 21041309 Free PMC article. - Distinct lipid effects on tBid and Bim activation of membrane permeabilization by pro-apoptotic Bax.
Shamas-Din A, Bindner S, Chi X, Leber B, Andrews DW, Fradin C. Shamas-Din A, et al. Biochem J. 2015 May 1;467(3):495-505. doi: 10.1042/BJ20141291. Biochem J. 2015. PMID: 25714678 - Apoptosis regulation at the mitochondrial outer membrane.
Gillies LA, Kuwana T. Gillies LA, et al. J Cell Biochem. 2014 Apr;115(4):632-40. doi: 10.1002/jcb.24709. J Cell Biochem. 2014. PMID: 24453042 Review. - Pro-apoptotic complexes of BAX and BAK on the outer mitochondrial membrane.
Wolf P, Schoeniger A, Edlich F. Wolf P, et al. Biochim Biophys Acta Mol Cell Res. 2022 Oct;1869(10):119317. doi: 10.1016/j.bbamcr.2022.119317. Epub 2022 Jun 22. Biochim Biophys Acta Mol Cell Res. 2022. PMID: 35752202 Review.
Cited by
- DNA damage-induced primordial follicle oocyte apoptosis and loss of fertility require TAp63-mediated induction of Puma and Noxa.
Kerr JB, Hutt KJ, Michalak EM, Cook M, Vandenberg CJ, Liew SH, Bouillet P, Mills A, Scott CL, Findlay JK, Strasser A. Kerr JB, et al. Mol Cell. 2012 Nov 9;48(3):343-52. doi: 10.1016/j.molcel.2012.08.017. Epub 2012 Sep 20. Mol Cell. 2012. PMID: 23000175 Free PMC article. - Proapoptotic Bak and Bax guard against fatal systemic and organ-specific autoimmune disease.
Mason KD, Lin A, Robb L, Josefsson EC, Henley KJ, Gray DH, Kile BT, Roberts AW, Strasser A, Huang DC, Waring P, O'Reilly LA. Mason KD, et al. Proc Natl Acad Sci U S A. 2013 Feb 12;110(7):2599-604. doi: 10.1073/pnas.1215097110. Epub 2013 Jan 24. Proc Natl Acad Sci U S A. 2013. PMID: 23349374 Free PMC article. - PUMA, a critical mediator of cell death--one decade on from its discovery.
Hikisz P, Kiliańska ZM. Hikisz P, et al. Cell Mol Biol Lett. 2012 Dec;17(4):646-69. doi: 10.2478/s11658-012-0032-5. Epub 2012 Sep 20. Cell Mol Biol Lett. 2012. PMID: 23001513 Free PMC article. Review. - Ryanodine receptors are targeted by anti-apoptotic Bcl-XL involving its BH4 domain and Lys87 from its BH3 domain.
Vervliet T, Lemmens I, Vandermarliere E, Decrock E, Ivanova H, Monaco G, Sorrentino V, Nadif Kasri N, Missiaen L, Martens L, De Smedt H, Leybaert L, Parys JB, Tavernier J, Bultynck G. Vervliet T, et al. Sci Rep. 2015 Apr 15;5:9641. doi: 10.1038/srep09641. Sci Rep. 2015. PMID: 25872771 Free PMC article. - Cephalotaxine Inhibits the Survival of Leukemia Cells by Activating Mitochondrial Apoptosis Pathway and Inhibiting Autophagy Flow.
Liu T, Guo Q, Zheng S, Liu Y, Yang H, Zhao M, Yao L, Zeng K, Tu P. Liu T, et al. Molecules. 2021 May 18;26(10):2996. doi: 10.3390/molecules26102996. Molecules. 2021. PMID: 34070111 Free PMC article.
References
- Chipuk JE, et al. Mitochondrial outer membrane permeabilization during apoptosis: the innocent bystander scenario. Cell Death Differ. 2006;13:1396–1402. - PubMed
- Riedl SJ, Salvesen GS. The apoptosome: signalling platform of cell death. Nat Rev Mol Cell Biol. 2007;8:405–413. - PubMed
- Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. - PubMed
- Newmeyer DD, et al. Cell-free apoptosis in Xenopus egg extracts: inhibition by Bcl-2 and requirement for an organelle fraction enriched in mitochondria. Cell. 1994;79:353–364. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials