Islet amyloid in type 2 diabetes, and the toxic oligomer hypothesis - PubMed (original) (raw)
Review
Islet amyloid in type 2 diabetes, and the toxic oligomer hypothesis
Leena Haataja et al. Endocr Rev. 2008 May.
Abstract
Type 2 diabetes (T2DM) is characterized by insulin resistance, defective insulin secretion, loss of beta-cell mass with increased beta-cell apoptosis and islet amyloid. The islet amyloid is derived from islet amyloid polypeptide (IAPP, amylin), a protein coexpressed and cosecreted with insulin by pancreatic beta-cells. In common with other amyloidogenic proteins, IAPP has the propensity to form membrane permeant toxic oligomers. Accumulating evidence suggests that these toxic oligomers, rather than the extracellular amyloid form of these proteins, are responsible for loss of neurons in neurodegenerative diseases. In this review we discuss emerging evidence to suggest that formation of intracellular IAPP oligomers may contribute to beta-cell loss in T2DM. The accumulated evidence permits the amyloid hypothesis originally developed for neurodegenerative diseases to be reformulated as the toxic oligomer hypothesis. However, as in neurodegenerative diseases, it remains unclear exactly why amyloidogenic proteins form oligomers in vivo, what their exact structure is, and to what extent these oligomers play a primary or secondary role in the cytotoxicity in what are now often called unfolded protein diseases.
Figures
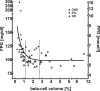
Figure 1
Relationship between percentage of pancreas volume occupied by β-cells and fasting plasma glucose in obese humans without insulin or oral antidiabetic treatment. The solid line is derived from nonlinear regression analysis (monoexponential fit, r = 0.50; P < 0.0001 by ANOVA). The dashed vertical lines indicate the mean β-cell area in obese nondiabetic subjects (OND) (right) and the computed inflection point of the curve (left). IFG, Impaired fasting glucose; OD, obese diabetic. Adapted from Ref. . [Copyright 2006 American Diabetes Association. From Diabetes Care 29:717–718. Reprinted with permission from The American Diabetes Association.]
Figure 2
Human islets from T2DM subjects (right) have less β-cells than those from nondiabetic subjects (left) and contain deposits of amyloid (arrow) derived from IAPP. Human islets were stained for insulin. This figure originally appeared in an article by Matveyenko and Butler (39). It is reprinted with permission from the ILAR Journal, Institute for Laboratory Animal Research, The National Academies (www.nationalacademies.org/ilar).
Figure 3
The islets from T2DM human (A), diabetic vervet monkey (B), and diabetic obese hemizygous hIAPP transgenic mouse (C) stained for amyloid using Congo red. (Unpublished images from the Butler laboratory.)
Figure 4
Alignment of IAPP ortholog proteins. Amino acid alignment of IAPP protein sequences identified in Homo sapiens (human, CAA39504), human mutant (S20G) (40), Macaca mulatto (monkey, XP_001098290), Felis catus (cat, NP_001036803), Canis lupus (dog, NP_001003233), Mus musculus (mouse, NP_034621), and Rattus norvegicus (rat, NP_036718). Dots correspond to conserved residues with human IAPP sequence. Red letters correspond to the amyloidic sequence.
Figure 5
The increased β-cell apoptosis in hemizygous hIAPP transgenic mice (OT) does not correspond to areas of amyloid. Islets from obese nontransgenic (ONT, left panels) and obese hemizygous hIAPP transgenic mice (OT, right panels) immunostained for insulin (upper panels) and corresponding islets stained for TUNEL (lower panels). Adapted from Ref. . [Copyright 2003 American Diabetes Association. From Diabetes 52:2304–2314. Reprinted with permission from The American Diabetes Association.]
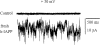
Figure 6
Stability of planar bilayer membranes is disrupted by addition of hIAPP. Control recording of bilayer capacitance and the same membrane 5 min after adding 10 μmol/liter freshly dissolved hIAPP to the cis chamber. Note membrane instability and increase in membrane electrical noise. Filtered at 0.3 kHz. Adapted from Ref. . [Copyright 1999 American Diabetes Association. From Diabetes 48:491–498. Reprinted with permission from The American Diabetes Association.]
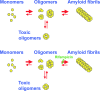
Figure 7
Proposed model for the balance between the different aggregation states of hIAPP in an aqueous solution. Once hIAPP oligomers are dissolved in an aqueous solution, IAPP intermediate structures (protofibrils) further assemble into amyloid fibrils. Alternatively, they may form toxic membrane-perforating toxic oligomers (top). In the presence of rifampicin, formation of amyloid fibrils is inhibited, but the formation of toxic oligomers is unaffected, consistent with continued cytotoxicity (bottom). This figure originally appeared in an article by J. J. Meier et al.: Am J Physiol Endocrinol Metab 291:E1317–E1324, 2006 (153). It is used with permission from the American Physiological Society.
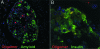
Figure 8
hIAPP toxic oligomers in islets from obese hemizygous hIAPP transgenic mice. hIAPP toxic oligomer immunoreactivity does not coincide with extracellular amyloid; it is predominantly intracellular, confined to β-cells, and it is perinuclear or in vesicle-like structures. Immunofluorescent staining for toxic hIAPP oligomers (red), autofluorescence for amyloid (green), and nuclei 4′,6-diamidino-2-phenylindole (DAPI) (blue) (A, 20× magnification). Immunofluorescent staining for toxic hIAPP oligomers (red), insulin (green), and nuclei DAPI (blue) (B, 100× magnification). Adapted from Ref. . [Copyright 2007 American Diabetes Association. From Diabetes 56:1324–1332. Reprinted with permission from The American Diabetes Association.]
Figure 9
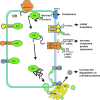
The schematic illustration of the UPR in protein secretory cells. Increased demand for BiP leads to detachment of BiP from PERK, IRE1α, and ATF6, which get activated. Activated PERK phosphorylates α-subunit of eukaryotic translation initiation factor 2 (eIF2α), which subsequently suppresses ER protein translation and leads to more ATF4. Activated IRE1α has a RNA editing function and removes the hairpin structure on inactive X-box-binding protein-1 (XBP1) mRNA (unspliced, u-XBP1), which later becomes active transcription factor (spliced, s-XBP1). Activated ATF6 translocates into the Golgi and undergoes partial intramembrane proteolysis by site-1 protease (S1P) and site-2 protease (S2P), then migrates to the nucleus. These three activated transcriptional factors then induce a series of responses to increase chaperone proteins, limit new protein translation, and increase the degradation of unfolded proteins (see Fig. 10 and Refs. ,,, and 131).
Figure 10
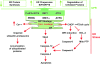
Proposed molecular signaling pathways of hIAPP-induced ER stress and apoptosis in pancreatic β-cells. BiP shortage activates three transcriptional factors (ATF4, ATF6, and XBP1), which collectively launch the UPR. In hIAPP overexpressing models and under insulin resistance conditions, an increased number of proteins in the ER leads to molecular crowding, which promotes protein aggregation and misfolding, especially of the amyloidogenic protein like hIAPP. Aggregated or unfolded hIAPP can compromise ER membrane barriers for ionic calcium. Decreased calcium inside the ER lumen and increased calcium in the cytosol may lead to ER stress, which is represented by nuclear translocation of CHOP, induction of death receptor DR5, down-regulation of BCL-2, cleavage of caspase-12, and accumulation of ubiquitinated proteins. Decreased ER calcium will decrease the efficiency of protein folding machinery and result in more unfolded proteins. Increased calcium in the cytosol may open up the mitochondrial permeability transition pore (Mito PTP), leading to cytochrome c release and caspase-9 activation (64,84,87,123,131).
Figure 11
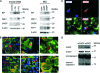
hIAPP induces ER stress responses in pancreatic β-cells. A, Immunoblotting of UPR and ER stress markers in islets from wild-type, rIAPP and hIAPP transgenic mice (r-TG and h-TG, respectively), and INS cells overexpressing GFP, rIAPP-EGFP, or hIAPP-EGFP. B, Nuclear translocation of CHOP (a–c); increased caspase-12 expression (d, e), and accumulation of polyubiquitinated proteins (Ubi-P, f) in islets from hIAPP transgenic mouse (a, d, f), hIAPP transgenic rat (HIP rat; b, e), and human islets from an obese T2DM subject (f). C, Nuclear CHOP is colocalized with the appearance of TUNEL staining in INS cells overexpressing hIAPP-EGFP. D, Knockdown of CHOP by small interfering RNA reduces the cleavage of caspase-3 in INS cells overexpressing hIAPP. This figure is adapted from the original figure that appeared in an article by C. J. Huang et al. (65,131). It is used with permission from The American Physiological Society. [Copyright 2007 American Diabetes Association. From Diabetes 56:2016–2027. Reprinted with permission from The American Diabetes Association.]
Similar articles
- Evidence for proteotoxicity in beta cells in type 2 diabetes: toxic islet amyloid polypeptide oligomers form intracellularly in the secretory pathway.
Gurlo T, Ryazantsev S, Huang CJ, Yeh MW, Reber HA, Hines OJ, O'Brien TD, Glabe CG, Butler PC. Gurlo T, et al. Am J Pathol. 2010 Feb;176(2):861-9. doi: 10.2353/ajpath.2010.090532. Epub 2009 Dec 30. Am J Pathol. 2010. PMID: 20042670 Free PMC article. - Diabetes due to a progressive defect in beta-cell mass in rats transgenic for human islet amyloid polypeptide (HIP Rat): a new model for type 2 diabetes.
Butler AE, Jang J, Gurlo T, Carty MD, Soeller WC, Butler PC. Butler AE, et al. Diabetes. 2004 Jun;53(6):1509-16. doi: 10.2337/diabetes.53.6.1509. Diabetes. 2004. PMID: 15161755 - Causative factors for formation of toxic islet amyloid polypeptide oligomer in type 2 diabetes mellitus.
Jeong HR, An SS. Jeong HR, et al. Clin Interv Aging. 2015 Nov 19;10:1873-9. doi: 10.2147/CIA.S95297. eCollection 2015. Clin Interv Aging. 2015. PMID: 26604727 Free PMC article. Review. - Human IAPP amyloidogenic properties and pancreatic β-cell death.
Fernández MS. Fernández MS. Cell Calcium. 2014 Nov;56(5):416-27. doi: 10.1016/j.ceca.2014.08.011. Epub 2014 Aug 27. Cell Calcium. 2014. PMID: 25224501 Review.
Cited by
- Circulating levels of IL-1B+IL-6 cause ER stress and dysfunction in islets from prediabetic male mice.
O'Neill CM, Lu C, Corbin KL, Sharma PR, Dula SB, Carter JD, Ramadan JW, Xin W, Lee JK, Nunemaker CS. O'Neill CM, et al. Endocrinology. 2013 Sep;154(9):3077-88. doi: 10.1210/en.2012-2138. Epub 2013 Jul 8. Endocrinology. 2013. PMID: 23836031 Free PMC article. - The Rationale for Insulin Therapy in Alzheimer's Disease.
Ribarič S. Ribarič S. Molecules. 2016 May 26;21(6):689. doi: 10.3390/molecules21060689. Molecules. 2016. PMID: 27240327 Free PMC article. Review. - Type 2 diabetes mellitus in adults: pathogenesis, prevention and therapy.
Lu X, Xie Q, Pan X, Zhang R, Zhang X, Peng G, Zhang Y, Shen S, Tong N. Lu X, et al. Signal Transduct Target Ther. 2024 Oct 2;9(1):262. doi: 10.1038/s41392-024-01951-9. Signal Transduct Target Ther. 2024. PMID: 39353925 Free PMC article. Review. - Development of a conditionally immortalized human pancreatic β cell line.
Scharfmann R, Pechberty S, Hazhouz Y, von Bülow M, Bricout-Neveu E, Grenier-Godard M, Guez F, Rachdi L, Lohmann M, Czernichow P, Ravassard P. Scharfmann R, et al. J Clin Invest. 2014 May;124(5):2087-98. doi: 10.1172/JCI72674. Epub 2014 Mar 25. J Clin Invest. 2014. PMID: 24667639 Free PMC article. - The role of FOXO1 in β-cell failure and type 2 diabetes mellitus.
Kitamura T. Kitamura T. Nat Rev Endocrinol. 2013 Oct;9(10):615-23. doi: 10.1038/nrendo.2013.157. Epub 2013 Aug 20. Nat Rev Endocrinol. 2013. PMID: 23959366 Review.
References
- Zimmet P, Alberti KG, Shaw J 2001 Global and societal implications of the diabetes epidemic. Nature 414:782–787 - PubMed
- Zeggini E, Weedon MN, Lindgren CM, Frayling TM, Elliott KS, Lango H, Timpson NJ, Perry JR, Rayner NW, Freathy RM, Barrett JC, Shields B, Morris AP, Ellard S, Groves CJ, Harries LW, Marchini JL, Owen KR, Knight B, Cardon LR, Walker M, Hitman GA, Morris AD, Doney AS, McCarthy MI, Hattersley AT 2007 Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science 316:1336–1341 - PMC - PubMed
- Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S, Balkau B, Heude B, Charpentier G, Hudson TJ, Montpetit A, Pshezhetsky AV, Prentki M, Posner BI, Balding DJ, Meyre D, Polychronakos C, Froguel P 2007 A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 445:881–885 - PubMed
- Jack Jr L, Boseman L, Vinicor F 2004 Aging Americans and diabetes. A public health and clinical response. Geriatrics 59:14–17 - PubMed
- Polonsky KS 2000 Dynamics of insulin secretion in obesity and diabetes. Int J Obes Relat Metab Disord 24(Suppl 2):S29–S31 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases