Two conventional protein kinase C isoforms, alpha and beta I, are involved in the ATP-induced activation of volume-regulated anion channel and glutamate release in cultured astrocytes - PubMed (original) (raw)
Comparative Study
Two conventional protein kinase C isoforms, alpha and beta I, are involved in the ATP-induced activation of volume-regulated anion channel and glutamate release in cultured astrocytes
Alena Rudkouskaya et al. J Neurochem. 2008.
Abstract
Volume-regulated anion channels (VRACs) are activated by cell swelling and are permeable to inorganic and small organic anions, including the excitatory amino acids glutamate and aspartate. In astrocytes, ATP potently enhances VRAC activity and glutamate release via a P2Y receptor-dependent mechanism. Our previous pharmacological study identified protein kinase C (PKC) as a major signaling enzyme in VRAC regulation by ATP. However, conflicting results obtained with potent PKC blockers prompted us to re-evaluate the involvement of PKC in regulation of astrocytic VRACs by using small interfering RNA (siRNA) and pharmacological inhibitors that selectively target individual PKC isoforms. In primary rat astrocyte cultures, application of hypoosmotic medium (30% reduction in osmolarity) and 20 microM ATP synergistically increased the release of excitatory amino acids, measured with a non-metabolized analog of L-glutamate, D-[(3)H]aspartate. Both Go6976, the selective inhibitor of Ca(2+)-sensitive PKCalpha, betaI/II, and gamma, and MP-20-28, a cell permeable pseudosubstrate inhibitory peptide of PKCalpha and betaI/II, reduced the effects of ATP on D-[(3)H]aspartate release by approximately 45-55%. Similar results were obtained with a mixture of siRNAs targeting rat PKCalpha and betaI. Surprisingly, down-regulation of individual alpha and betaI PKC isozymes by siRNA was completely ineffective. These data suggest that ATP regulates VRAC activity and volume-sensitive excitatory amino acid release via cooperative activation of PKCalpha and betaI.
Figures
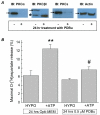
Fig. 1. The conventional PKC inhibitor Go6976 and the membrane-permeable PKCα/β inhibitory peptide (MP20-28) reduce the ATP-induced stimulation of the excitatory amino acid release from swollen rat astrocytes
A, Primary astrocyte cultures were loaded with
d
-[3H]aspartate overnight, washed from the extracellular isotope, and then superfused with isoosmotic or hypoosmotic media as indicated. Go6976 (1 μM) was added 20 min before and during application of hypoosmotic medium. 20 μM ATP was added to hypoosmotic medium only. Data are the mean values ±SE of 5-6 experiments in each group. ***, p<0.001, ATP vs. all other groups; ###, p<0.001 ATP+Go6976 vs. all other groups. B, Similar experiment performed with the inhibitory peptide MP20-28. For clarity, only maximal release values under hypoosmotic conditions are presented. Cells were pretreated with 10 μM MP20-28 for 30 min before the efflux experiment. The peptide was not present in the release assay media. Data are means ±SE of 5-6 experiments in each group. *p<0.05, ***p<0.001, vs. hypoosmotic control. #p=0.05, ATP vs. ATP+MP20-28.
Fig. 2. Phorbol ester-induced PKC downregulation strongly inhibits the effect of ATP on swelling-activated d-[3H]aspartate release
A, Representative Western blots showing downregulation of conventional PKCs α and βI, and novel PKCε in astrocyte cultures treated with the phorbol ester PDBu (500 nM) for 24 hrs in serum-free opti-MEM. Actin immunoreactivity in the same cell lysate is shown as a protein loading control. Arrows indicate positions of the predicted molecular weights for PKCα (~82 kD), PKC βI (~79kD), PKCε (~95 kD) and actin (~42 kD). * marks likely product of proteolytic degradation of PKC βI. B, Maximal
d
-[3H]aspartate release values in cultured astrocytes exposed to hypoosmotic medium in the presence/absence of 20 μM ATP. Cells were maintained in opti-MEM for 24 hrs in the presence/absence of 500 nM PDBu as indicated. Data are the mean values ±SE of 5 experiments in each group. **p=0.01 vs. hypoosmotic control, #p<0.05 vs. hypoosmotic control in the PDBu-treated cells.
Fig. 3. Representative micrographs showing primary astrocyte cultures transfected with scrambled siRNA labeled with Alexa-488
A, Nomarski DIC optic image of siRNA-transfected cultured astrocytes, 72 hrs after transfection. B, The same optical field showing Alexa-488 fluorescence captured with FITC set of filters. Florescent signal was largely restricted to puncta surrounding nuclei; dim fluorescent signal in cytosol is not seen because of the difference in the signal intensities. C, Overlap of DIC and fluorescence images. All images were captured on Olympus IX-71 fluorescence microscope at 16× magnification.
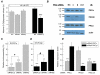
Fig. 4. siRNA-induced downregulation of conventional PKC isoforms α and βI attenuates the effect of ATP on swelling-activated excitatory amino acid release
A, Representative Western blots showing the effect of the siRNA mix targeting PKCα and βI on the PKCα and PKCβI protein levels at 24-72 hrs post-transfection. NC, negative control, cells treated with the scrambled siRNA for 72 hrs. Actin staining in a stripped blot is shown as a loading control. Bars and numbers on the right show positions of molecular weight markers. B, The effect of PKCα and βI downregulation on the ATP-induced
d
-[3H]aspartate release from swollen astrocytes. Cells were treated with siRNA mix for 72 hrs. Scrambled siRNA was used as a negative control (NC). Three independent siRNA transfections were performed. Data represent the mean values ±SE of 5-6 experiments in each group. *** p<0.001, ATP NC vs. HYPO NC. ### p<0.01, siRNA treatment significantly decreased
d
-[3H]aspartate release in the presence of ATP.
Fig. 5. Independent downregulation of PKCα or βI does not affect the ATP-induced d-[3H]aspartate release from swollen cells
A, Effect of siRNA transfections targeting PKCα or βI on the swelling-activated
d
-[3H]aspartate release in the presence of ATP. The results were always compared to the matching negative controls (NC) treated with scrambled siRNA on the same day. The experimental design was identical to that presented in Fig. 4B. For clarity, only the maximal release values are presented. Data are the mean values ±SE of 5 experiments in each group. For comparison, the effect of α/βI siRNA mix is shown on the right (the data are taken from the experiments presented in Fig. 4B). ***p<0.001 vs. NC. B, Representative Western Blots showing changes in the PKC isozyme levels 72 hrs after transfection of astrocytes with siRNA-PKCα (α), siRNA-PKC βI (β), or their combination (α/β). Immunostaining of PKCε and actin was used to control for the specificity of the siRNA effects and protein loading. Arrows and numbers on the left show positions of molecular weight markers. C, Quantified changes in the PKCα immunoreactivity in cells treated with siRNA targeting PKCα, βI, or α+βI. Protein lysates from cultures treated with siRNA for 72 hrs were subjected to Western blot analysis using a monoclonal anti-PKCα antibody. The integral density of an immunoreactive band of ~82 kD was compared to the PKC band in protein lysates from cells treated with scrambled siRNA. Mean normalized optical densities ±SE of 8 independent protein preparations are shown. ***p<0.001 vs. scrambled siRNA, #p<0.05 siRNAα vs. siRNAα/βI. D, Quantified changes in the PKCβI immunoreactivity in cells treated with siRNA targeting PKCα, βI, or α+βI. Protein lysates were analyzed using monoclonal anti-PKCβI antibody recognizing the band of ~80 kD. Mean normalized optical densities ±SE of 4 independent protein preparations are shown. ***p<0.05, **p<0.01 vs. scrambled siRNA. *p<0.05 vs. scrambled siRNA. #p<0.01 siRNAα vs. siRNAα/βI. E, Quantitative RT-PCR analysis of the mRNA levels for PKCα and PKCβI in astrocyte cultures treated for 24 hrs with siRNA targeting PKCα, PKCβI, or their combination. All message levels were normalized to the levels of GAPDH in the same samples. Data are the mean values of 3 independent qRT-PCR analyses performed for two independent siRNA transfections (n=6). *p<0.05, ***p<0.001 vs. scrambled siRNA control.
Similar articles
- ATP regulates anion channel-mediated organic osmolyte release from cultured rat astrocytes via multiple Ca2+-sensitive mechanisms.
Mongin AA, Kimelberg HK. Mongin AA, et al. Am J Physiol Cell Physiol. 2005 Jan;288(1):C204-13. doi: 10.1152/ajpcell.00330.2004. Epub 2004 Sep 15. Am J Physiol Cell Physiol. 2005. PMID: 15371260 - ATP potently modulates anion channel-mediated excitatory amino acid release from cultured astrocytes.
Mongin AA, Kimelberg HK. Mongin AA, et al. Am J Physiol Cell Physiol. 2002 Aug;283(2):C569-78. doi: 10.1152/ajpcell.00438.2001. Am J Physiol Cell Physiol. 2002. PMID: 12107067 - Activation of microglia with zymosan promotes excitatory amino acid release via volume-regulated anion channels: the role of NADPH oxidases.
Harrigan TJ, Abdullaev IF, Jourd'heuil D, Mongin AA. Harrigan TJ, et al. J Neurochem. 2008 Sep;106(6):2449-62. doi: 10.1111/j.1471-4159.2008.05553.x. Epub 2008 Jul 9. J Neurochem. 2008. PMID: 18624925 Free PMC article. - Anion channels in astrocytes: biophysics, pharmacology, and function.
Kimelberg HK, MacVicar BA, Sontheimer H. Kimelberg HK, et al. Glia. 2006 Nov 15;54(7):747-757. doi: 10.1002/glia.20423. Glia. 2006. PMID: 17006903 Free PMC article. Review.
Cited by
- Calcium is not required for triggering volume restoration in hypotonically challenged A549 epithelial cells.
Ponomarchuk O, Boudreault F, Orlov SN, Grygorczyk R. Ponomarchuk O, et al. Pflugers Arch. 2016 Nov;468(11-12):2075-2085. doi: 10.1007/s00424-016-1896-4. Epub 2016 Oct 31. Pflugers Arch. 2016. PMID: 27796579 - Receptor regulation of osmolyte homeostasis in neural cells.
Fisher SK, Heacock AM, Keep RF, Foster DJ. Fisher SK, et al. J Physiol. 2010 Sep 15;588(Pt 18):3355-64. doi: 10.1113/jphysiol.2010.190777. Epub 2010 May 24. J Physiol. 2010. PMID: 20498228 Free PMC article. Review. - Structure-function relationships of the LRRC8 subunits and subdomains of the volume-regulated anion channel (VRAC).
Ghouli MR, Fiacco TA, Binder DK. Ghouli MR, et al. Front Cell Neurosci. 2022 Aug 10;16:962714. doi: 10.3389/fncel.2022.962714. eCollection 2022. Front Cell Neurosci. 2022. PMID: 36035259 Free PMC article. Review. - Trends in volume-regulated anion channel (VRAC) research: visualization and bibliometric analysis from 2014 to 2022.
Liu T, Li Y, Wang D, Stauber T, Zhao J. Liu T, et al. Front Pharmacol. 2023 Jul 19;14:1234885. doi: 10.3389/fphar.2023.1234885. eCollection 2023. Front Pharmacol. 2023. PMID: 37538172 Free PMC article. - Volume-dependent osmolyte efflux from neural tissues: regulation by G-protein-coupled receptors.
Fisher SK, Cheema TA, Foster DJ, Heacock AM. Fisher SK, et al. J Neurochem. 2008 Sep;106(5):1998-2014. doi: 10.1111/j.1471-4159.2008.05510.x. Epub 2008 Jun 2. J Neurochem. 2008. PMID: 18518929 Free PMC article. Review.
References
- Banderali U, Roy G. Anion channels for amino-acids in Mdck cells. Am J Physiol. 1992;263:C1200–C1207. - PubMed
- Berdiev BK, Xia J, Jovov B, Markert JM, Mapstone TB, Gillespie GY, Fuller CM, Bubien JK, Benos DJ. Protein kinase C isoform antagonism controls BNaC2 (ASIC1) function. J Biol Chem. 2002;277:45734–45740. - PubMed
- Brodie C, Kuperstein I, Acs P, Blumberg PM. Differential role of specific PKC isoforms in the proliferation of glial cells and the expression of the astrocytic markers GFAP and glutamine synthetase. Brain Res Mol Brain Res. 1998;56:108–117. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous