mGluR-dependent long-term depression is associated with increased phosphorylation of S6 and synthesis of elongation factor 1A but remains expressed in S6K-deficient mice - PubMed (original) (raw)
mGluR-dependent long-term depression is associated with increased phosphorylation of S6 and synthesis of elongation factor 1A but remains expressed in S6K-deficient mice
Marcia D Antion et al. Mol Cell Biol. 2008 May.
Abstract
Metabotropic glutamate receptor-dependent long-term depression (mGluR-LTD) in the hippocampus requires rapid protein synthesis, which suggests that mGluR activation is coupled to signaling pathways that regulate translation. Herein, we have investigated the signaling pathways that couple group I mGluRs to ribosomal S6 protein phosphorylation and 5'oligopyrimidine tract (5'TOP)-encoded protein synthesis during mGluR-LTD. We found that mGluR-LTD was associated with increased phosphorylation of p70S6 kinase (S6K1) and S6, as well as the synthesis of the 5'TOP-encoded protein elongation factor 1A (EF1A). Moreover, we found that LTD-associated increases in S6K1 phosphorylation, S6 phosphorylation, and levels of EF1A were sensitive to inhibitors of phosphoinositide 3-kinase (PI3K), mammalian target of rapamycin (mTOR), and extracellular signal-regulated kinase (ERK). However, mGluR-LTD was normal in S6K1 knockout mice and enhanced in both S6K2 knockout mice and S6K1/S6K2 double knockout mice. In addition, we observed that LTD-associated increases in S6 phosphorylation were still increased in S6K1- and S6K2-deficient mice, whereas basal levels of EF1A were abnormally elevated. Taken together, these findings indicate that mGluR-LTD is associated with PI3K-, mTOR-, and ERK-dependent alterations in the phosphorylation of S6 and S6K. Our data also suggest that S6Ks are not required for the expression of mGluR-LTD and that the synthesis of 5'TOP-encoded proteins is independent of S6Ks during mGluR-LTD.
Figures
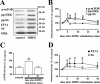
FIG. 1.
mGluR-LTD is associated with transient phosphorylation of mTOR, ERK, and ribosomal protein S6 in hippocampal area CA1 that is accompanied by enhanced synthesis of the 5′TOP-encoded proteins EF1A and S6. Hippocampal slices were exposed to DHPG (50 μM) for 10 min and incubated in ACSF for the indicated times. (A) Representative Western blots from the same experiment for phosphorylated mTOR (p-mTOR), phosphorylated ERK (pp-ERK), phosphorylated S6 (pp-S6), EF1A, total S6 (S6), and total ERK (ERK) from untreated (control) and DHPG-treated slices. Numbers on the right indicate molecular size markers. (B and D) After DHPG stimulation, slices were harvested immediately after treatment (0 min) and 5, 15, 30, and 60 min thereafter. Immunoreactivity was normalized to total ERK and graphed as percent control. Values are means ± standard errors of the means determined immediately after DHPG stimulation. (B) Percentages of control values are as follows: for pp-S6 with DHPG, 132% ± 10% (n = 7); for p-mTOR with DHPG, 115% ± 5% (n = 5); and for pp-ERK with DHPG, 138% ± 17% (n = 5). *, statistical significance above control for all groups; #, statistical significance above control for pp-S6 and pp-ERK. (C) After DHPG stimulation, slices were assayed immediately (0 min) or 10 min later for mTOR phosphotransferase activity. Percentages of control values are as follows: for mTOR activity with DHPG (0 min), 144% ± 11% (n = 7), and for DHPG (10 min), 97% ± 9% (n = 5). *, statistical significance above control mTOR activity levels. (D) Percentage of control EF1A value for DHPG is 122% ± 7% (n = 7), and percentage of control value for total S6 values with DHPG is 138% ± 17% (n = 3). *, statistical significance above control for all groups; #, statistical significance above control for S6.
FIG. 2.
mGluR-LTD is associated with increased phosphorylated S6 and EF1A levels in area CA1. Representative confocal images in soma and dendrites of hippocampal area CA1 from DHPG-treated and untreated slices (control). To determine whether there was elevated immunoreactivity in the dendrites and/or the soma of area CA1, the optical densities of the images were quantified in 20-μm intervals from the interface of the stratum pyramidale (s.p.) and stratum radiatum (s.r.) in control and DHPG-treated slices for pp-S6 (n = 4) and EF1A (n = 4). Elevations of pp-S6 and EF1A levels were detected in the s.p. but not in the s.r. with this method. Statistics were computed with a two-way ANOVA; post hoc Bonferroni t tests revealed significance (*, P < 0.05; **, P < 0.001). Images were taken with a 20× objective. Bar, 20 μm.
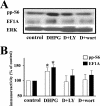
FIG. 3.
mGluR-LTD is associated with a PI3K-dependent increase in the levels of phosphorylated ribosomal protein S6 and total levels of the 5′TOP-encoded protein EF1A. Hippocampal slices were incubated in vehicle (0.001% DMSO plus 0.01% methanol), LY292002 (LY) (50 μM), or wortmannin (wort) (100 nm) for 30 min and were then exposed to DHPG (50 μM) for 10 min in the presence of either vehicle (control) or the respective antagonists. (A) Representative Western blots for phosphorylated S6 (pp-S6), EF1A, and total ERK. (B) Immunoreactivity was normalized to total ERK immunoreactivity. Values are means ± standard errors of the means and are expressed as percentages of control values. pp-S6 values are as follows: with DHPG, 132% ± 11% (n = 8); with DHPG plus LY (D+LY), 97% ± 20% (n = 8); and with DHPG plus wort (D+wort), 103% ± 18% (n = 5) (data not shown: with LY, 97% ± 10% [n = 8]; with wort, 110% ± 16% [n = 5]). EF1A values are as follows: with DHPG, 144% ± 9% (n = 4); with DHPG plus LY, 118% ± 19% (n = 4); and with DHPG plus wort, 119% ± 15% (n = 4) (data not shown: with LY, 120% ± 14% [n = 4]; with wort, 97% ± 14% [n = 4]). *, statistical significance with a Student t test (P < 0.05).
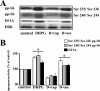
FIG. 4.
mGluR-LTD is associated with mTOR-dependent increases in the levels of phosphorylated ribosomal protein S6 and total levels of EF1A. Hippocampal slices were incubated in vehicle (0.01% methanol), rapamycin (rap) (20 nM), a selective antagonist for mTOR, or ascomycin (asc) (20 nM), a molecule structurally similar to rapamycin that does not interfere with mTOR activity, for 30 min and were then exposed to DHPG (50 μM) for 10 min in the presence of vehicle (control) or the respective antagonists. (A) Representative Western blots for S6 phosphorylated at Ser235/Ser236 and Ser240/Ser244 (pp-S6), EF1A, and total ERK. (B) Immunoreactivity was normalized to total ERK immunoreactivity. Values are means ± standard errors of the means and are expressed as percentages of control values. Ser235/Ser236 pp-S6 values are as follows: with DHPG, 138% ± 10% (n = 6); with DHPG plus rap (D+rap), 73% ± 8% (n = 5); and with DHPG plus asc (D+asc), 128% ± 13% (n = 5) (data not shown: with rap, 91% ± 6% [n = 4]; with asc, 92% ± 8% [n = 4]). Ser240/Ser244 pp-S6 values are as follows: with DHPG, 144% ± 15% (n = 4); with DHPG plus rap, 80% ± 5% (n = 3); and with DHPG plus asc, 111% ± 2% (n = 3) (data not shown: with rap, 89% ± 1% [n = 2]; with asc, 89% ± 8% [n = 2]). EF1A values are as follows: with DHPG, 122% ± 11% (n = 4); with DHPG plus rap, 79% ± 16% (n = 4); and with DHPG plus asc, 113% ± 7% (n = 4) (data not shown: with rap, 79% ± 23% [n = 4]; with asc, 86% ± 28% [n = 4]). *, statistical significance with control via Student's t test (P < 0.05).
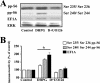
FIG. 5.
mGluR-LTD is associated with an ERK-dependent increase in the levels of phosphorylated ribosomal protein S6 and total levels of EF1A. Hippocampal slices were incubated in vehicle (0.01% DMSO), UO126 (20 μM), a selective antagonist for the ERK1/2-specific MEK, or SL327 (5 μM), a structurally distinct antagonist from UO126 for the ERK1/2-specific MEK, for 60 min and were then exposed to DHPG (50 μM) for 10 min in the presence of vehicle (control) or the respective antagonists. (A) Representative Western blots for S6 phosphorylated at Ser235/Ser236 and Ser240/Ser244 (pp-S6), EF1A, and ERK. (B) Immunoreactivity was normalized to total ERK immunoreactivity. Values are means ± standard errors of the means and are expressed as percentages of control values. Ser235/Ser236 pp-S6 values are as follows: with DHPG, 127% ± 14% (n = 7), and with DHPG plus UO126 (D+UO126), 84% ± 9% (n = 7) (data not shown: with UO126, 90% ± 13% [n = 6]; with DHPG plus SL327, 90% ± 4% [n = 4]; with SL327, 99% ± 8% [n = 4]). Ser240/Ser244 pp-S6 values are as follows: with DHPG, 164% ± 15% (n = 4), and with DHPG plus UO126, 101% ± 8% (n = 4) (data not shown: with UO126, 91% ± 15% [n = 4]; with DHPG plus SL327, 92% ± 8% [n = 4]; with SL327, 99% ± 5% [n = 4]). EF1A values are as follows: with DHPG, 194% ± 31% (n = 4), and with DHPG plus UO126, 109% ± 20% (n = 4) (data not shown: with UO126, 120% ± 26% [n = 4]; with DHPG plus SL327, 137% ± 50% [n = 4]; with SL327, 115% ± 31% [n = 4]). *, statistical significance with control via Student's t test (P < 0.05).
FIG. 6.
DHPG induces phosphorylation of S6K that requires ERK, PI3K, and mTOR signaling pathways. Hippocampal slices were incubated in vehicle (0.01% DMSO or 0.01% methanol), UO126 (20 μM, 1 h), rapamycin (rap) (20 nM, 30 min), or LY294002 (LY) (50 μM, 30 min) and were then exposed to DHPG (50 μM) for 10 min in the presence of vehicle (control) or the respective antagonists. (A) Representative immunoreactivity for ERK-dependent phosphorylation of Thr421/Ser424 p70S6K, Thr444/Ser447 of p85S6K, and total p70/p85S6K in hippocampal CA1 homogenates. (B) Thr421/Ser424 phosphorylated p70S6K immunoreactivity was normalized to total p70S6K immunoreactivity. Values are means ± standard errors of the means. Percentages of control values for 421/424 p70S6K (pp-p70S6K) are as follows: with DHPG, 170% ± 21% (n = 8), and with DHPG plus UO126 (D+UO126), 65% ± 15% (n = 4) (data not shown: with DHPG plus LY, 70% ± 30% [n = 4]; with DHPG plus rap, 84% ± 28% [n = 4]). Phosphorylated p85S6K immunoreactivity could not be densitometrically quantified, as control-treated levels were not observed in most experiments. (C) Representative immunoreactivity for mTOR- and PI3K-dependent phosphorylation of Thr389 p70S6K, Thr412 p85S6K, and total p70/p85S6K in hippocampal CA1 homogenates. (D) Thr389 phosphorylated p70S6K immunoreactivity was normalized to total p70S6K immunoreactivity. Values are means ± standard errors of the means. Percentages of control values for Thr389 p70S6K (pp-70S6K) are as follows: with DHPG, 140% ± 13% (n = 8); with DHPG plus rap (D+rap), 95% ± 13% (n = 4); and with DHPG plus LY (D+LY), 105% ± 21% (n = 4) (data not shown: with DHPG plus UO126, 124% ± 12% [n = 4]). *, statistical significance with vehicle-treated slices via Student's t test (P ≤ 0.05).
FIG. 7.
mGluR-LTD in hippocampal slices from S6K knockout mice. (A, B, and D) mGluR-LTD was induced by incubation with DHPG (50 μM) for 10 min in slices from wild-type (WT), S6K1 knockout (S6K1 KO), S6K2 knockout (S6K2 KO), and S6K1/S6K2 double knockout (dKO) mice. For all recordings, at least 20 min of basal fEPSPs was recorded prior to LTD induction with DHPG. Representative fEPSPs 10 min before (a) and 45 min after (b) DHPG treatment in WT and KO slices are depicted on the top of each panel. (A) Ensemble averages for the initial slope of the fEPSP 20 min before, 10 min during, and 50 min after treatment with DHPG in WT and S6K1 KO hippocampal slices are shown at the bottom of the panel. For WT mice, eight slices were taken from seven animals, t = 40 to 50 min, and the fEPSP slope was 77% ± 9% of baseline; for S6K1 KO mice, eight slices were taken from seven animals, t = 40 to 50 min, and the fEPSP slope was 65% ± 7% of baseline. (B) Ensemble averages for the initial slope of the fEPSP 20 min before, 10 min during, and 50 min after treatment with DHPG in WT and S6K2 KO hippocampal slices are shown at the bottom of the panel. For WT mice, eight slices were taken from seven animals, t = 40 to 50 min, and the fEPSP slope was 82% ± 5% of baseline; for S6K2 KO mice, eight slices were taken from seven animals, t = 40 to 50 min, and the fEPSP slope was 61% ± 5% of baseline. (C) S6K1 levels in hippocampal homogenates from WT, S6K1 KO, and S6K2 KO mice. A representative Western blot is shown in the top panel, with the p85S6K and p70S6K isoforms of S6K1 indicated on the left. In the bottom panel, p70S6K1 immunoreactivity of each mutant animal was normalized to WT p70S6K1 immunoreactivity. (D) Ensemble averages for the initial slope of the fEPSP 20 min before, 10 min during, and 50 min after treatment with DHPG in WT and dKO hippocampal slices are shown. For WT mice, six slices were taken from three animals, t = 40 to 50 mine, and the fEPSP slope was 87% ± 7% of baseline; for dKO mice, six slices were taken from three animals, t = 40 to 50 min, and the fEPSP slope was 41% ± 8% of baseline. (E) mGluR-LTD was measured in slices from S6K2 KO or WT littermates in the presence of 40 μM anisomycin 1 h prior to, during, and 10 min after DHPG (50 μM for 10 min) treatment. Representative fEPSPs 10 min before (a) and 90 min after (b) DHPG treatment are depicted at the top of the panel. Ensemble averages for the initial slope of the fEPSP 20 min before, 10 min during, and 120 min after treatment with DHPG in WT and S6K2 KO hippocampal slices are shown at the bottom of the panel. For WT mice, five slices were taken from five animals, t = 60 to 80 min, and the fEPSP slope was 95% ± 9% of baseline (P > 0.1 compared to t = −20 to 0 min); for S6K2 KO mice, eight slices were taken from five animals, t = 60 to 80 min, and the fEPSP slope was 66% ± 12% of baseline (P < 0.01 compared to t = −20 to 0 min). (F) Comparison of LTD in WT, S6K1 KO, S6K2 KO, and dKO mice. Values were calculated 40 to 50 min after the start of DHPG treatment. For WT mice, the fEPSP slope was 82% ± 4% of baseline (21 slices); for S6K1 KO mice, the fEPSP slope was 74% ± 6% of baseline (10 slices); for S6K2 KO mice, the fEPSP slope was 62% ± 4% of baseline (12 slices); and for dKO mice, the fEPSP slope was 41% ± 8% of baseline (6 slices). Statistics were calculated with a one-way ANOVA, with a P value of <0.05 (*) and a P value of <0.001 (**) indicating significance compared to values for WT mice.
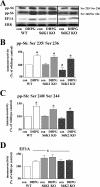
FIG. 8.
mGluR-LTD in slices from S6K knockout mice is associated with normal phosphorylation of ribosomal protein S6 and abnormal expression of the 5′TOP-encoded protein EF1A. Hippocampal slices from wild-type (WT), S6K1 knockout (S6K1 KO), and S6K2 knockout (S6K2 KO) mice were incubated in DHPG (50 μM) for 10 min. (A) Representative Western blot from the same experiment within multiple gels for S6 phosphorylated at Ser235/Ser236 and Ser240/Ser244 (pp-S6), EF1A, and total ERK. (B to D) Immunoreactivity was normalized to total ERK immunoreactivity. Values are means ± standard errors of the means and are expressed as percentages of levels for the WT control (con). (B) Ser235/Ser236 pp-S6 values are as follows: for WT mice with DHPG, 136% ± 7% (n = 5); for S6K1 KO mice with control, 101% ± 5% (n = 4); for S6K1 KO mice with DHPG, 131% ± 6% (n = 4); for S6K2 KO mice with control, 79% ± 10% (n = 3); and for S6K2 KO mice with DHPG, 124% ± 4% (n = 3). (C) Ser240/Ser244 pp-S6 values are as follows: for WT mice with DHPG, 140% ± 8% (n = 5); for S6K1 KO mice with control, 101% ± 3% (n = 4); for S6K1 KO mice with DHPG, 128% ± 5% (n = 4); for S6K2 KO mice with control, 91% ± 5% (n = 3); and for S6K2 KO mice with DHPG, 121% ± 3% (n = 3). (D) EF1A values are as follows: for WT mice with DHPG, 120% ± 5% (n = 5); for S6K1 KO mice with control, 134% ± 11% (n = 5); for S6K1 KO mice with DHPG, 137% ± 11% (n = 5); for S6K2 KO mice with control, 121% ± 6% (n = 3); and for S6K2 KO mice with DHPG, 124% ± 5% (n = 3). *, statistical significance above control; #, statistical significance below WT control by Student's t test (P < 0.05).
Similar articles
- S6K1(-/-)/S6K2(-/-) mice exhibit perinatal lethality and rapamycin-sensitive 5'-terminal oligopyrimidine mRNA translation and reveal a mitogen-activated protein kinase-dependent S6 kinase pathway.
Pende M, Um SH, Mieulet V, Sticker M, Goss VL, Mestan J, Mueller M, Fumagalli S, Kozma SC, Thomas G. Pende M, et al. Mol Cell Biol. 2004 Apr;24(8):3112-24. doi: 10.1128/MCB.24.8.3112-3124.2004. Mol Cell Biol. 2004. PMID: 15060135 Free PMC article. - Necessary, but not sufficient: insights into the mechanisms of mGluR mediated long-term depression from a rat model of early life seizures.
Bernard PB, Castano AM, Bayer KU, Benke TA. Bernard PB, et al. Neuropharmacology. 2014 Sep;84:1-12. doi: 10.1016/j.neuropharm.2014.04.011. Epub 2014 Apr 26. Neuropharmacology. 2014. PMID: 24780380 Free PMC article. - Role of S6 phosphorylation and S6 kinase in cell growth.
Volarević S, Thomas G. Volarević S, et al. Prog Nucleic Acid Res Mol Biol. 2001;65:101-27. doi: 10.1016/s0079-6603(00)65003-1. Prog Nucleic Acid Res Mol Biol. 2001. PMID: 11008486 Review. - Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks.
Magnuson B, Ekim B, Fingar DC. Magnuson B, et al. Biochem J. 2012 Jan 1;441(1):1-21. doi: 10.1042/BJ20110892. Biochem J. 2012. PMID: 22168436 Review.
Cited by
- Genetic removal of p70 S6 kinase 1 corrects molecular, synaptic, and behavioral phenotypes in fragile X syndrome mice.
Bhattacharya A, Kaphzan H, Alvarez-Dieppa AC, Murphy JP, Pierre P, Klann E. Bhattacharya A, et al. Neuron. 2012 Oct 18;76(2):325-37. doi: 10.1016/j.neuron.2012.07.022. Epub 2012 Oct 17. Neuron. 2012. PMID: 23083736 Free PMC article. - Calmodulin activity regulates group I metabotropic glutamate receptor-mediated signal transduction and synaptic depression.
Sethna F, Zhang M, Kaphzan H, Klann E, Autio D, Cox CL, Wang H. Sethna F, et al. J Neurosci Res. 2016 May;94(5):401-8. doi: 10.1002/jnr.23719. Epub 2016 Feb 11. J Neurosci Res. 2016. PMID: 26864654 Free PMC article. - Characterizing autism spectrum disorders by key biochemical pathways.
Subramanian M, Timmerman CK, Schwartz JL, Pham DL, Meffert MK. Subramanian M, et al. Front Neurosci. 2015 Sep 24;9:313. doi: 10.3389/fnins.2015.00313. eCollection 2015. Front Neurosci. 2015. PMID: 26483618 Free PMC article. Review. - Translational control mechanisms in long-lasting synaptic plasticity and memory.
Gkogkas C, Sonenberg N, Costa-Mattioli M. Gkogkas C, et al. J Biol Chem. 2010 Oct 15;285(42):31913-7. doi: 10.1074/jbc.R110.154476. Epub 2010 Aug 6. J Biol Chem. 2010. PMID: 20693284 Free PMC article. Review. No abstract available. - G Protein-Coupled Receptors As Regulators of Localized Translation: The Forgotten Pathway?
Tréfier A, Pellissier LP, Musnier A, Reiter E, Guillou F, Crépieux P. Tréfier A, et al. Front Endocrinol (Lausanne). 2018 Feb 2;9:17. doi: 10.3389/fendo.2018.00017. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 29456523 Free PMC article. Review.
References
- Bardoni, B., and J. L. Mandel. 2002. Advances in understanding of fragile X pathogenesis and FMRP function, and in identification of X linked mental retardation genes. Curr. Opin. Genet. Dev. 12284-293. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS034007/NS/NINDS NIH HHS/United States
- R01 NS047384/NS/NINDS NIH HHS/United States
- NS047384/NS/NINDS NIH HHS/United States
- R37 NS034007/NS/NINDS NIH HHS/United States
- R29 NS034007/NS/NINDS NIH HHS/United States
- NS034007/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous