New weapons and a rapid response against insect attack - PubMed (original) (raw)
Review
New weapons and a rapid response against insect attack
John Browse et al. Plant Physiol. 2008 Mar.
No abstract available
Figures
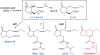
Figure 1.
Biosynthesis and metabolism of JA. The octadecanoid pathway converts 18:3 to (+)-7-_iso_-JA, which epimerizes to the more stable (−)-JA isomer. It is generally assumed that (+)-7-_iso_-JA is the biologically relevant and more active form of the hormone. JA is metabolized to a wide range of derivatives (see text for details). For example, JA carboxyl methyltransferase (JMT in the image) converts JA to the volatile compound MeJA. Conjugation of JA to Ile by JAR1 produces JA-Ile, which promotes COI1 interaction with the JAZ1 repressor protein. Other enzymes, presumably related to JAR1, catalyze the formation of other JA-amino acid conjugates (JACs in the image). Coronatine (in red) is a phytotoxin produced by virulent strains of Pseudomonas syringae. The toxin functions as a JA mimic and is structurally related to JA-Ile.
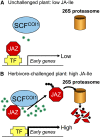
Figure 2.
Model of JA signaling in response to herbivore attack. A, Low intracellular levels of JA-Ile (green stars) favor the accumulation of JAZ proteins, which repress the activity of transcription factors (TF in the image) such as MYC2 that positively regulate JA-responsive genes. B, Tissue injury caused by insect herbivores results in rapid accumulation of bioactive JAs, which promote SCFCOI1-mediated ubiquitination and subsequent degradation of JAZ proteins via the 26S proteasome. JA-induced removal of JAZ proteins causes derepression of TF and the activation of early response genes. Although this model depicts JA-Ile as the active signal for triggering JAZ degradation, current evidence does not exclude the possibility that other JAs are also active. See text for more details.
Similar articles
- Why does herbivore attack reconfigure primary metabolism?
Schwachtje J, Baldwin IT. Schwachtje J, et al. Plant Physiol. 2008 Mar;146(3):845-51. doi: 10.1104/pp.107.112490. Plant Physiol. 2008. PMID: 18316639 Free PMC article. Review. No abstract available. - Recognition of herbivory-associated molecular patterns.
Mithöfer A, Boland W. Mithöfer A, et al. Plant Physiol. 2008 Mar;146(3):825-31. doi: 10.1104/pp.107.113118. Plant Physiol. 2008. PMID: 18316636 Free PMC article. Review. No abstract available. - Jasmonate signalling in plants shapes plant-insect interaction ecology.
Lortzing T, Steppuhn A. Lortzing T, et al. Curr Opin Insect Sci. 2016 Apr;14:32-39. doi: 10.1016/j.cois.2016.01.002. Epub 2016 Jan 25. Curr Opin Insect Sci. 2016. PMID: 27436644 Review. - Cross talk in defense signaling.
Koornneef A, Pieterse CM. Koornneef A, et al. Plant Physiol. 2008 Mar;146(3):839-44. doi: 10.1104/pp.107.112029. Plant Physiol. 2008. PMID: 18316638 Free PMC article. Review. No abstract available. - Arthropod-inducible proteins: broad spectrum defenses against multiple herbivores.
Zhu-Salzman K, Luthe DS, Felton GW. Zhu-Salzman K, et al. Plant Physiol. 2008 Mar;146(3):852-8. doi: 10.1104/pp.107.112177. Plant Physiol. 2008. PMID: 18316640 Free PMC article. Review. No abstract available.
Cited by
- Crosstalk between GA and JA signaling mediates plant growth and defense.
Hou X, Ding L, Yu H. Hou X, et al. Plant Cell Rep. 2013 Jul;32(7):1067-74. doi: 10.1007/s00299-013-1423-4. Epub 2013 Mar 24. Plant Cell Rep. 2013. PMID: 23525761 Review. - Transcriptional and metabolic insights into the differential physiological responses of arabidopsis to optimal and supraoptimal atmospheric CO2.
Kaplan F, Zhao W, Richards JT, Wheeler RM, Guy CL, Levine LH. Kaplan F, et al. PLoS One. 2012;7(8):e43583. doi: 10.1371/journal.pone.0043583. Epub 2012 Aug 20. PLoS One. 2012. PMID: 22916280 Free PMC article. - Jasmonate signaling: a conserved mechanism of hormone sensing.
Katsir L, Chung HS, Koo AJ, Howe GA. Katsir L, et al. Curr Opin Plant Biol. 2008 Aug;11(4):428-35. doi: 10.1016/j.pbi.2008.05.004. Epub 2008 Jun 24. Curr Opin Plant Biol. 2008. PMID: 18583180 Free PMC article. Review. - The chloroplast-localized phospholipases D α4 and α5 regulate herbivore-induced direct and indirect defenses in rice.
Qi J, Zhou G, Yang L, Erb M, Lu Y, Sun X, Cheng J, Lou Y. Qi J, et al. Plant Physiol. 2011 Dec;157(4):1987-99. doi: 10.1104/pp.111.183749. Epub 2011 Oct 7. Plant Physiol. 2011. PMID: 21984727 Free PMC article. - Induction of Salt Tolerance in Arabidopsis thaliana by Volatiles From Bacillus amyloliquefaciens FZB42 via the Jasmonic Acid Signaling Pathway.
Liu S, Tian Y, Jia M, Lu X, Yue L, Zhao X, Jin W, Wang Y, Zhang Y, Xie Z, Wang R. Liu S, et al. Front Microbiol. 2020 Nov 12;11:562934. doi: 10.3389/fmicb.2020.562934. eCollection 2020. Front Microbiol. 2020. PMID: 33281760 Free PMC article.
References
- Balbi V, Devoto A (2008) Jasmonate signalling network in Arabidopsis thaliana: crucial regulatory nodes and new physiological scenarios. New Phytol 177 301–318 - PubMed
- Bodenhausen N, Reymond P (2007) Signaling pathways controlling induced resistance to insect herbivores in Arabidopsis. Mol Plant Microbe Interact 20 1406–1420 - PubMed
- Browse J (2005) Jasmonate: an oxylipin signal with many roles in plants. Vitam Horm 72 431–456 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources