Distribution and expression of soluble epoxide hydrolase in human brain - PubMed (original) (raw)
Distribution and expression of soluble epoxide hydrolase in human brain
Priyanka Sura et al. J Histochem Cytochem. 2008 Jun.
Abstract
Epoxyeicosatrienoic acids (EETs) are cytochrome P450 metabolites of arachidonic acid, which function in the brain to regulate cerebral blood flow and protect against ischemic brain injury. EETs are converted by soluble epoxide hydrolase (sEH) to the corresponding inactive diol metabolites. Previous animal studies have indicated that sEH gene deletion or treatment with sEH inhibitors results in increased levels of EETs and protection against stroke-induced brain damage. To begin elucidating the underlying mechanism for these effects, we sought to determine the distribution, expression, and activity of sEH in human brain samples obtained from patients with no neurological changes/pathologies. Immunohistochemical analyses showed the distribution of sEH mainly in the neuronal cell bodies, oligodendrocytes, and scattered astrocytes. Surprisingly, in the choroid plexus, sEH was found to be highly expressed in ependymal cells. Vascular localization of sEH was evident in several regions, where it was highly expressed in the smooth muscles of the arterioles. Western blot analysis and enzyme assays confirmed the presence of sEH in the normal brain. Our results indicate differential localization of sEH in the human brain, thus suggestive of an essential role for this enzyme in the central nervous system. This manuscript contains online supplemental material at http://www.jhc.org. Please visit this article online to view these materials.
Figures
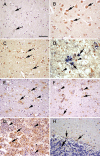
Figure 1
Soluble epoxide hydrolase (sEH) immunoreactivity (brown) in sections of human brain. (A) Occipital lobe, negative control with no primary antibody. No immunoreactivity was present in the neurons (arrows). (B) Occipital lobe. There is diffuse sEH immunoreactivity of the neuronal cell bodies (arrows). (C) Parietal lobe. sEH immunoreactivity was observed in scattered glial cells (arrows). (D) Parietal lobe, double immunolabeling for sEH (brown) and GFAP (blue). Note there is colocalization of blue over brown (arrows) and some glial cells with sEH immunoreactivity alone (arrowheads). (E) White matter, immunoreactivity of sEH in the white matter, neuropil (arrowhead), and occasional staining of glial cells (arrows). (F) Substantia nigra. sEH immunoreactivity was observed in glial cells (arrows). Note the presence of dark brown granular neuromelanin in the cytoplasm of neurons (arrowheads). (G) Pituitary. There is marked sEH immunoreactivity in acidophils and chromophobes (arrows). However, there is no staining in basophils (arrowheads). (H) Cerebellum. There is scattered sEH immunoreactivity in the granular cell layer and molecular cell layer glia (arrows). Bar = 120 μm.
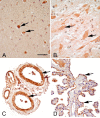
Figure 2
sEH immunoreactivity (brown) in medulla, spinal cord, meningeal blood vessels, and choroid plexus. (A) Medulla oblongata, immunoreactivity of sEH in the neuronal cell bodies of medulla (arrows). (B) Spinal cord. sEH immunoreactivity was observed in neuronal cell bodies of spinal cord (arrows). (C) Meningeal blood vessels. There is intense immunoreactivity of the endothelial cells and the smooth muscles of arteries and arterioles (arrows). (D) Choroid plexus. The ependymal cells (arrows) lining the choroid plexus are immunoreactive. Bar = 120 μm.
Figure 3
Western blot analysis of sEH expression in the human brain. There is sEH expression in the human frontal lobe, occipital lobe, parietal lobe, temporal lobe, and thalamus. His-tagged purified human sEH (hsEH) and human liver are used as positive controls. β-actin was used as a loading control for brain tissue samples.
Similar articles
- Distribution of soluble and microsomal epoxide hydrolase in the mouse brain and its contribution to cerebral epoxyeicosatrienoic acid metabolism.
Marowsky A, Burgener J, Falck JR, Fritschy JM, Arand M. Marowsky A, et al. Neuroscience. 2009 Oct 6;163(2):646-61. doi: 10.1016/j.neuroscience.2009.06.033. Epub 2009 Jun 18. Neuroscience. 2009. PMID: 19540314 - Characterization of transgenic mice with neuron-specific expression of soluble epoxide hydrolase.
Bianco RA, Agassandian K, Cassell MD, Spector AA, Sigmund CD. Bianco RA, et al. Brain Res. 2009 Sep 29;1291:60-72. doi: 10.1016/j.brainres.2009.07.060. Epub 2009 Jul 28. Brain Res. 2009. PMID: 19643090 Free PMC article. - Soluble epoxide hydrolase gene deletion is protective against experimental cerebral ischemia.
Zhang W, Otsuka T, Sugo N, Ardeshiri A, Alhadid YK, Iliff JJ, DeBarber AE, Koop DR, Alkayed NJ. Zhang W, et al. Stroke. 2008 Jul;39(7):2073-8. doi: 10.1161/STROKEAHA.107.508325. Epub 2008 Mar 27. Stroke. 2008. PMID: 18369166 Free PMC article. - Epoxyeicosatrienoic acids and soluble epoxide hydrolase in physiology and diseases of the central nervous system.
Kuo YM, Lee YH. Kuo YM, et al. Chin J Physiol. 2022 Jan-Feb;65(1):1-11. doi: 10.4103/cjp.cjp_80_21. Chin J Physiol. 2022. PMID: 35229747 Review. - Humble beginnings with big goals: Small molecule soluble epoxide hydrolase inhibitors for treating CNS disorders.
Zarriello S, Tuazon JP, Corey S, Schimmel S, Rajani M, Gorsky A, Incontri D, Hammock BD, Borlongan CV. Zarriello S, et al. Prog Neurobiol. 2019 Jan;172:23-39. doi: 10.1016/j.pneurobio.2018.11.001. Epub 2018 Nov 14. Prog Neurobiol. 2019. PMID: 30447256 Free PMC article. Review.
Cited by
- Inhibiting the soluble epoxide hydrolase increases the EpFAs and ERK1/2 expression in the hippocampus of LiCl-pilocarpine post-status epilepticus rat model.
Peng W, Hu Z, Shen Y, Wang X. Peng W, et al. IBRO Neurosci Rep. 2024 Oct 11;17:329-336. doi: 10.1016/j.ibneur.2024.10.001. eCollection 2024 Dec. IBRO Neurosci Rep. 2024. PMID: 39492986 Free PMC article. - Inhibition of Soluble Epoxide Hydrolase Ameliorates Cerebral Blood Flow Autoregulation and Cognition in Alzheimer's Disease and Diabetes-Related Dementia Rat Models.
Tang C, Border JJ, Zhang H, Gregory A, Bai S, Fang X, Liu Y, Wang S, Hwang SH, Gao W, Morgan GC, Smith J, Bunn D, Cantwell C, Wagner KM, Morisseau C, Yang J, Shin SM, O'Herron P, Bagi Z, Filosa JA, Dong Y, Yu H, Hammock BD, Roman RJ, Fan F. Tang C, et al. bioRxiv [Preprint]. 2024 Aug 31:2024.08.30.610540. doi: 10.1101/2024.08.30.610540. bioRxiv. 2024. PMID: 39257786 Free PMC article. Preprint. - Inhibition of Soluble Epoxide Hydrolase by Cembranoid Diterpenes from Soft Coral Sinularia maxima: Enzyme Kinetics, Molecular Docking, and Molecular Dynamics.
Phong NV, Thao NP, Vinh LB, Luyen BTT, Minh CV, Yang SY. Phong NV, et al. Mar Drugs. 2024 Aug 17;22(8):373. doi: 10.3390/md22080373. Mar Drugs. 2024. PMID: 39195489 Free PMC article. - Interaction between α-Synuclein and Bioactive Lipids: Neurodegeneration, Disease Biomarkers and Emerging Therapies.
Sanluca C, Spagnolo P, Mancinelli R, De Bartolo MI, Fava M, Maccarrone M, Carotti S, Gaudio E, Leuti A, Vivacqua G. Sanluca C, et al. Metabolites. 2024 Jun 22;14(7):352. doi: 10.3390/metabo14070352. Metabolites. 2024. PMID: 39057675 Free PMC article. Review. - Regulation of soluble epoxide hydrolase in renal-associated diseases: insights from potential mechanisms to clinical researches.
Gao P, Cao Y, Ma L. Gao P, et al. Front Endocrinol (Lausanne). 2024 Feb 15;15:1304547. doi: 10.3389/fendo.2024.1304547. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38425758 Free PMC article. Review.
References
- Al-Anizy M, Horley NJ, Kuo CW, Gillett LC, Laughton CA, Kendall D, Barrett DA, et al. (2006) Cytochrome P450 Cyp4x1 is a major P450 protein in mouse brain. FEBS J 273:936–947 - PubMed
- Alkayed NJ, Birks EK, Narayanan J, Petrie KA, Kohler-Cabot AE, Harder DR (1997) Role of P-450 arachidonic acid epoxygenase in the response of cerebral blood flow to glutamate in rats. Stroke 28:1066–1072 - PubMed
- Alkayed NJ, Narayanan J, Gebremedhin D, Medhora M, Roman RJ, Harder DR (1996) Molecular characterization of an arachidonic acid epoxygenase in rat brain astrocytes. Stroke 27:971–979 - PubMed
- Amruthesh SC, Falck JR, Ellis EF (1992) Brain synthesis and cerebrovascular action of epoxygenase metabolites of arachidonic acid. J Neurochem 58:503–510 - PubMed
- Bjorkhem I, Meaney S (2004) Brain cholesterol: long secret life behind a barrier. Arterioscler Thromb Vasc Biol 24:806–815 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources