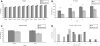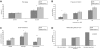A partial loss of function allele of methyl-CpG-binding protein 2 predicts a human neurodevelopmental syndrome - PubMed (original) (raw)
. 2008 Jun 15;17(12):1718-27.
doi: 10.1093/hmg/ddn062. Epub 2008 Mar 4.
Affiliations
- PMID: 18321864
- PMCID: PMC2666042
- DOI: 10.1093/hmg/ddn062
A partial loss of function allele of methyl-CpG-binding protein 2 predicts a human neurodevelopmental syndrome
Rodney C Samaco et al. Hum Mol Genet. 2008.
Abstract
Rett Syndrome, an X-linked dominant neurodevelopmental disorder characterized by regression of language and hand use, is primarily caused by mutations in methyl-CpG-binding protein 2 (MECP2). Loss of function mutations in MECP2 are also found in other neurodevelopmental disorders such as autism, Angelman-like syndrome and non-specific mental retardation. Furthermore, duplication of the MECP2 genomic region results in mental retardation with speech and social problems. The common features of human neurodevelopmental disorders caused by the loss or increase of MeCP2 function suggest that even modest alterations of MeCP2 protein levels result in neurodevelopmental problems. To determine whether a small reduction in MeCP2 level has phenotypic consequences, we characterized a conditional mouse allele of Mecp2 that expresses 50% of the wild-type level of MeCP2. Upon careful behavioral analysis, mice that harbor this allele display a spectrum of abnormalities such as learning and motor deficits, decreased anxiety, altered social behavior and nest building, decreased pain recognition and disrupted breathing patterns. These results indicate that precise control of MeCP2 is critical for normal behavior and predict that human neurodevelopmental disorders will result from a subtle reduction in MeCP2 expression.
Figures
Figure 1.
The expression of Mecp2 is decreased in Mecp2 Flox/y mice at both the mRNA and the protein level. The mRNA level as measured by qRT-PCR (A) is decreased in mutant animals. Notably, the e1 isoform (measured by a probe spanning the exons 1–3 boundary, e1–3), the e2 isoform (measured by a probe spanning exons 2–3 boundary, e2–3) and the common exon 3 (probe e3) all are decreased by ∼50% in _Mecp2_Flox/y mice compared with wild-type littermates. The overall protein levels of MeCP2 measured by western blot (B) are decreased in _Mecp2_Flox/y animals compared with wild-type littermates. MeCP2 was detected by an antibody specific to the common carboxy-terminus (α-C-term). The bottom blot in (B) is a loading control probed with an antibody to Gapdh. Each lane represents biological replicates of the respective genotypes. The decrease in MeCP2 protein level is also observed by immunofluorescence (C, D). Whereas the expression of MeCP2 is clearly discernable as relatively bright nuclear foci in wild-type animals (C), in _Mecp2_Flox/y animals the signal is weaker (D). The small dashed box shows the cortical region represented at higher magnification in the inset in (C) and (D). The higher magnification reveals decreased MeCP2 levels, but a similar cellular distribution of MeCP2. The scale bar in the large figure in (C) represents 1 mm and within the inset represents 10 µm.
Figure 2.
Mecp2_Flox/y_ mice are heavier and perform poorly on motor tasks. _Mecp2_Flox/y mice (F1 129S6.B6, n = 16 for each genotype) are ∼1 g heavier than littermate wild-type (WT) controls, although this finding is only significant at 9 and 16 weeks of life (A). The mice perform poorly on a variety of motor tasks including accelerating rotarod (B), dowel walking (C and D) and wire hanging (C). On the accelerating rotarod, the mutant mice perform poorly on Day 1, which indicates an inherent coordination deficit. The _Mecp2_Flox/y mice fall off the wire sooner than controls (C, P = 0.002, Mann–Whitney) but not on the dowel (P = 0.12, Mann–Whitney). Additionally, the _Mecp2_Flox/y animals have fewer side touches in both the dowel task (D, P = 0.013 Mann–Whitney) and the wire hang task (not shown, P = 0.002 Mann–Whitney). *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 3.
Mecp2_Flox/y_ mice have altered pain sensitivity, prepulse inhibition, social interactions and nest building. _Mecp2_Flox/y mice (F1 129S6.B6, n = 16 for each genotype) have increased latency of paw withdrawal on the hotplate (A, P = 0.002); however, they show normal response to tail flick at 40 (A), 50 and 60°C (not shown). The _Mecp2_Flox/y animals have a diminished startle response (not shown, P = 0.001) but increased inhibitory gating when presented with a 74 or 82 dB sound prepulse (B). _Mecp2_Flox/y mice (F1 129.B6, n = 16 for each genotype) spent a greater percentage of time at the partition than WT littermate controls when both an unfamiliar mouse was placed in the adjacent chamber or when the mice were re-exposed to a familiar mouse (C, *P < 0.05, **P < 0.01). When singly housed with a fresh nesting material, many _Mecp2_Flox/y mice (n = 15) did not show any attempt to form a nest and only a small percentage had a fully formed nest after 14 h, in contrast to the large percentage (D, χ2 P = 0.02) of WT littermate control animals (n = 16).
Figure 4.
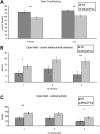
Mecp2_Flox/y_ mice have altered learning and decreased anxiety. The _Mecp2_Flox/y mice (F1 129S6.FVB, n = 16 per genotype) have decreased learning in a fear conditioning task both when exposed to the context (A, P = 0.006) or to the cue stimulus (A, P = 0.004). Additionally, they appear to be less anxious as measured by the distance traveled within the center of an open field chamber compared with the total distance (B) during the first and third 10 min intervals. This decreased anxiety is also apparent by the increased vertical exploratory movements that the mutant animals under took during the first and third 10 min intervals in the open field chamber (C). *P < 0.05, **P < 0.01.
Figure 5.
Respiration is altered in Mecp2 Flox/y mice. Representative plethysmographic recordings showing the irregular rhythm and periods of apnea in F1 129S6.FVB _Mecp2_Flox/y mice (B) relative to WT mice (A). Population data showing the incidence of apneas (C, n = 4 for each genotype). Population data showing the coefficients of variability of respiratory frequency (D, n = 4 for each genotype). *P < 0.05.
Similar articles
- Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes.
Chao HT, Chen H, Samaco RC, Xue M, Chahrour M, Yoo J, Neul JL, Gong S, Lu HC, Heintz N, Ekker M, Rubenstein JL, Noebels JL, Rosenmund C, Zoghbi HY. Chao HT, et al. Nature. 2010 Nov 11;468(7321):263-9. doi: 10.1038/nature09582. Nature. 2010. PMID: 21068835 Free PMC article. - Postnatal loss of methyl-CpG binding protein 2 in the forebrain is sufficient to mediate behavioral aspects of Rett syndrome in mice.
Gemelli T, Berton O, Nelson ED, Perrotti LI, Jaenisch R, Monteggia LM. Gemelli T, et al. Biol Psychiatry. 2006 Mar 1;59(5):468-76. doi: 10.1016/j.biopsych.2005.07.025. Epub 2005 Sep 30. Biol Psychiatry. 2006. PMID: 16199017 - Learning and memory and synaptic plasticity are impaired in a mouse model of Rett syndrome.
Moretti P, Levenson JM, Battaglia F, Atkinson R, Teague R, Antalffy B, Armstrong D, Arancio O, Sweatt JD, Zoghbi HY. Moretti P, et al. J Neurosci. 2006 Jan 4;26(1):319-27. doi: 10.1523/JNEUROSCI.2623-05.2006. J Neurosci. 2006. PMID: 16399702 Free PMC article. - The role of MeCP2 in brain development and neurodevelopmental disorders.
Gonzales ML, LaSalle JM. Gonzales ML, et al. Curr Psychiatry Rep. 2010 Apr;12(2):127-34. doi: 10.1007/s11920-010-0097-7. Curr Psychiatry Rep. 2010. PMID: 20425298 Free PMC article. Review. - MeCP2 dysfunction in humans and mice.
Zoghbi HY. Zoghbi HY. J Child Neurol. 2005 Sep;20(9):736-40. doi: 10.1177/08830738050200090701. J Child Neurol. 2005. PMID: 16225828 Review.
Cited by
- Sex-specific Behavioral Features of Rodent Models of Autism Spectrum Disorder.
Jeon SJ, Gonzales EL, Mabunga DFN, Valencia ST, Kim DG, Kim Y, Adil KJL, Shin D, Park D, Shin CY. Jeon SJ, et al. Exp Neurobiol. 2018 Oct;27(5):321-343. doi: 10.5607/en.2018.27.5.321. Epub 2018 Oct 31. Exp Neurobiol. 2018. PMID: 30429643 Free PMC article. Review. - Exploration of group II metabotropic glutamate receptor modulation in mouse models of Rett syndrome and MECP2 Duplication syndrome.
Vermudez SAD, Buch A, Weiss K, Gogliotti RG, Niswender CM. Vermudez SAD, et al. Neuropharmacology. 2022 May 15;209:109022. doi: 10.1016/j.neuropharm.2022.109022. Epub 2022 Mar 3. Neuropharmacology. 2022. PMID: 35248529 Free PMC article. - Sensory processing in autism spectrum disorders and Fragile X syndrome-From the clinic to animal models.
Sinclair D, Oranje B, Razak KA, Siegel SJ, Schmid S. Sinclair D, et al. Neurosci Biobehav Rev. 2017 May;76(Pt B):235-253. doi: 10.1016/j.neubiorev.2016.05.029. Epub 2016 May 24. Neurosci Biobehav Rev. 2017. PMID: 27235081 Free PMC article. Review. - MeCP2 co-ordinates liver lipid metabolism with the NCoR1/HDAC3 corepressor complex.
Kyle SM, Saha PK, Brown HM, Chan LC, Justice MJ. Kyle SM, et al. Hum Mol Genet. 2016 Jul 15;25(14):3029-3041. doi: 10.1093/hmg/ddw156. Epub 2016 Jun 10. Hum Mol Genet. 2016. PMID: 27288453 Free PMC article. - Long-lived epigenetic interactions between perinatal PBDE exposure and Mecp2308 mutation.
Woods R, Vallero RO, Golub MS, Suarez JK, Ta TA, Yasui DH, Chi LH, Kostyniak PJ, Pessah IN, Berman RF, LaSalle JM. Woods R, et al. Hum Mol Genet. 2012 Jun 1;21(11):2399-411. doi: 10.1093/hmg/dds046. Epub 2012 Feb 15. Hum Mol Genet. 2012. PMID: 22343140 Free PMC article.
References
- Hagberg B., Aicardi J., Dias K., Ramos O. A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls: Rett's syndrome: report of 35 cases. Ann. Neurol. 1983;14:471–479. - PubMed
- Neul J.L., Zoghbi H.Y. Rett syndrome: a prototypical neurodevelopmental disorder. Neuroscientist. 2004;10:118–128. - PubMed
- Nan X., Campoy F.J., Bird A. MeCP2 is a transcriptional repressor with abundant binding sites in genomic chromatin. Cell. 1997;88:471–481. - PubMed
- Nan X., Cross S., Bird A. Gene silencing by methyl-CpG-binding proteins. Novartis Found. Symp. 1998;214:6–16. Discussion 16–21, 46–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS052240/NS/NINDS NIH HHS/United States
- NS057819/NS/NINDS NIH HHS/United States
- HD024064/HD/NICHD NIH HHS/United States
- P30 HD024064/HD/NICHD NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- K08 NS052240/NS/NINDS NIH HHS/United States
- AS1622/AS/Autism Speaks/United States
- R01 HD024234/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases