Neurodegeneration and motor dysfunction in a conditional model of Parkinson's disease - PubMed (original) (raw)
Comparative Study
. 2008 Mar 5;28(10):2471-84.
doi: 10.1523/JNEUROSCI.3040-07.2008.
Elisabeth Petrasch-Parwez, Beate Winner, Jürgen Winkler, Stephan von Hörsten, Thorsten Schmidt, Jana Boy, Melanie Kuhn, Huu P Nguyen, Peter Teismann, Jörg B Schulz, Manuela Neumann, Bernd J Pichler, Gerald Reischl, Carsten Holzmann, Ina Schmitt, Antje Bornemann, Wilfried Kuhn, Frank Zimmermann, Antonio Servadio, Olaf Riess
Affiliations
- PMID: 18322092
- PMCID: PMC6671187
- DOI: 10.1523/JNEUROSCI.3040-07.2008
Comparative Study
Neurodegeneration and motor dysfunction in a conditional model of Parkinson's disease
Silke Nuber et al. J Neurosci. 2008.
Abstract
Alpha-synuclein (alpha-syn) has been implicated in the pathogenesis of many neurodegenerative disorders, including Parkinson's disease. These disorders are characterized by various neurological and psychiatric symptoms based on progressive neuropathological alterations. Whether the neurodegenerative process might be halted or even reversed is presently unknown. Therefore, conditional mouse models are powerful tools to analyze the relationship between transgene expression and progression of the disease. To explore whether alpha-syn solely originates and further incites these alterations, we generated conditional mouse models by using the tet-regulatable system. Mice expressing high levels of human wild-type alpha-syn in midbrain and forebrain regions developed nigral and hippocampal neuropathology, including reduced neurogenesis and neurodegeneration in absence of fibrillary inclusions, leading to cognitive impairment and progressive motor decline. Turning off transgene expression in symptomatic mice halted progression but did not reverse the symptoms. Thus, our data suggest that approaches targeting alpha-syn-induced pathological pathways might be of benefit rather in early disease stages. Furthermore, alpha-syn-associated cytotoxicity is independent of filamentous inclusion body formation in our conditional mouse model.
Figures
Figure 1.
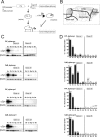
Conditional mouse design and expression pattern of human α-syn in brains of tg mice. A, tTA expression was either driven by the PrP or the CaM promoter. In its active form, tTA binds to the tetO7 sequence of the minimal promoter and initiates expression of the downstream localized human α_-syn_ gene. Expression can be inhibited by the presence of dox, because it binds to tTA, making it inactive. B, Subregional brain dissection for Western blot analysis. The reconstructed atlas template is according to the stereotaxic atlas (Paxinos and Franklin, 2001). C, Western blot analyses (20 μg per lane) of human α-syn in dissected tg mouse brains using the 15G7-α-syn antibody and β-actin as internal loading control. After administration of dox for 3 weeks (gene off), reduced protein levels in adult double-tg mice of each line were observed. Human brain was used as positive control. D, Band intensities were quantified using ImageQuant software and normalized to the β-actin staining in the same line to correct for variations in loading. After normalization, the amount of human α-syn was calculated in percentage relative to expression intensity of the human brain signal. +, Unspecific background staining of membrane. Ob, Olfactory bulb; Cx, cortex; Bg, basal ganglia; Ce, cerebellum; Bs, brainstem; Hu, human brain.
Figure 2.
Distribution of human α-syn expression in treated and untreated conditional tg mice. Sagittal sections showed regional immunostaining for human α-syn in the brain of adult untreated CaM_α-syn mice (A–C) and PrP_α-syn mice respectively (D–F). A, Strong immunoreactivity was detected in the olfactory bulb (Ob), olfactory tubercle (Tu), cortex (Cx), caudate–putamen (CPu), globus pallidus (GP), substantia nigra (SN), thalamus (Th), and hippocampus (Hc) in CaM_α-syn mice. B, In the CA1 area of the Hc, α-syn immunoreactivity was observed in a subpopulation of the pyramidal cells (PY) (arrows), various dendrites in the stratum radiatum (RAD) (arrowhead), and in the neuropil of the molecular layer (mol). C, Intense α-syn staining was observed in both the substantia nigra pars compacta (pc) and pars reticulata (pr). D, PrP_α-syn mice displayed minor expression in the Ob, the CPu, the GP, the Hc, and the pr in comparison with CaM_α-syn mice. Additional staining was observed in the cerebellum (Ce). E, Expression of α-syn was detected in the molecular layer (MOL) and in the granular layer (GL), without staining of the Purkinje cell layer (P). F, Staining was prominent in the pc but less expressed in the pr. Doxycycline treatment over a 3 week period of adult CaM_α-syn mice (G–I) and adult PrP_α-syn mice (J–L) partly ceased transgene expression. H, I, In CaM_α-syn mice, treatment resulted in complete loss of immunoreactivity in the stratum oriens (OR), PY, and RAD of Hc, whereas the mol, the overlying Cx (H), and neuronal processes of the SN (I) were still stained, although less prominent. J–L, In treated PrP_α-syn mice, residual α-syn expression was detected in the Th (J) but abolished in the GL (not in the MOL) of the Ce (K) and reduced in the pc and pr of the SN (L). α-Syn staining was missing in CaM_α-syn mice (M–O) and PrP_α-syn mice (P–R), when mice were born and raised (over a minimum of 8 weeks) with dox. Scale bar: B, C, E, H, I, K, N, O, Q, R, 20 μm; A, D, G, J, M, P, 200 μm; F, L, 5 μm.
Figure 3.
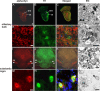
Confocal images of α-syn and TH double-staining immunofluorescence and electron microscopy of α-syn expression in the olfactory bulb and the substantia nigra. Frontal brain sections of 20-month-old CaM_α-syn mice were double-labeled against α-syn (red) and TH (green) to detect transgene expression in dopaminergic structures of the olfactory bulb (A–H) and substantia nigra (I–P). A, α-syn staining was detected in the granular (GrO) and, to a minor extent, in the glomerular layer (Gl) of the olfactory bulb; the latter contains also TH-positive structures (B). C, Merged image shows a partial overlap of α-syn expression and TH in the Gl. E, F, Higher magnification of the Gl revealed prominent punctate α-syn immunostaining of the neuropil (arrows) (E) and TH immunoreaction also in neuronal somata (F). G, Merged image shows no colocalization of α-syn and TH-positive neurons, but a moderate colocalization in the neuropil. D, H, Electron microscopy of the Gl confirmed α-syn accumulation in the neuropil localized in unmyelinated axons (D, arrows) and synaptic terminals (T) (H). I, In the SN, α-syn was most prominent in the pars reticulata (pr) and less expressed in the pars compacta (pc), which is comprised of TH-positive cells (J). K, Merged image illustrated overlapping of α-syn and TH staining in the intersectional area between pc and pr. M, Higher magnification of this area detected α-syn in neuronal somata with accumulations varying in size, in some cases being colocalized with TH (N) as clearly identified in the merged image (O, arrow). L, Electron microscopy of the intersectional area revealed α-syn in myelinated (X) and unmyelinated (arrows) axons. P, Semithin section confirmed different accumulation sizes of α-syn in neuronal somata (arrow) and in fiber bundles (FB). Scale bar: A–C, I–K, 1 mm; E–G, M–O, 100 μm; D, H, L, 500 nm; P, 1 μm.
Figure 4.
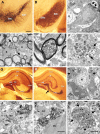
Immunoelectron microscopy of neurodegeneration and associated α-syn in 20-month-old CaM_α-syn mice in the substantia nigra and hippocampus. A, TH-stained vibratome section showed dopaminergic neurons in the substantia nigra pars compacta (SNc) and their cell processes extending toward the pars reticulata (SNr). B, Adjacent section displayed strong 15G7-α-syn immunoreactivity in the SNc and SNr. C, The nigral neuron (DC) neighbored to a glial cell (G) localized at the border of the SNc to the SNr showed signs of dark cell degeneration. The nucleus appeared slightly collapsed and the cytoplasm darkened with pronounced accumulation of lysosomes (arrows). D, Large lipid droplets (x) were dispersed among the fiber bundles of the SNr closely associated with immunopositive unmyelinated nerve fibers (arrows). E, Several myelinated axons (AX) contained dark condensed organelles, also observed in unmyelinated structures (F) associated with α-syn immunopositive patches (arrows). G, Calbindin immunostaining showed the different subareas of the hippocampus. H, The adjacent α-syn-stained section exhibited labeling of the pyramidal cell layer in the CA1, the stratum lacunosum moleculare (LM), and the inner molecular layer (IML) of the dentate gyrus. Prominent α-syn immunoreactivity was obvious in the curved stripe of mossy fibers and terminals in the CA3 area sharply contrasting to the lightly stained hilus (H) of the dentate gyrus. I, Dark degenerated neurons (DC) (for localization, see arrow in H) were dispersed among normal neurons (N). The dark mossy fiber bundles (white “x”), frequently positive for α-syn immunoreactivity, were localized between the proximal dendrites (x), some of which exhibited signs of degeneration (arrows). J, K, Many α-syn-positive mossy fiber terminals (MT) formed asymmetric contacts with immunonegative dendritic spines (SP) (J) or were associated with DC (K). L, Dystrophic structures (arrows) with condensed organelles were also identified in the dentate gyrus. Scale bar: A, B, 100 μm; C, 1 μm; D–F, 200 nm; G, H, 100 μm; I, 4 μm; J, K, 500 nm; L, 2 μm.
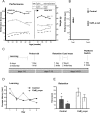
Figure 5.
Nigral cell death in CaM_α-syn mice. A, B, Toluidine blue-stained sections of a 9-month-old control mouse (A) and a CaM_α-syn littermate (B) showed numerous dark cells (arrows) in the pars reticulata (SNr) in the tg α-syn mouse that were absent in the control. Various dark cells were also distributed among healthy neurons in the pars compacta (SNc). C, D, Higher enlarged pictures of the pars reticulata of the control showed the large lightly stained healthy neurons (C) and the shrunken dark degenerated cells (arrows) in the respective area of the CaM α-syn mouse (D). F, Single apoptotic cells (arrows) were detected in CaM_α-syn mice, were absent in control mice (E). G, Graph showing stereological counting of TH-positive SN neurons revealed a strong tendency of reduction, bordering significant level (p = 0.07). Error bars indicate SEM. H, Counting of dark cells in two CaM α-syn mice revealed a high amount of dark cells (DCD) in the substantia nigra pars reticulata and a minor amount of dark cells in the pars compacta. In both controls, dark cells were missing.
Figure 6.
Effect of α-syn expression on neurotransmitter content in brain of old-aged untreated (gene on) and treated (gene off) conditional mice. A–C, Content of neurotransmitter was measured in the olfactory bulb (A), striatum (B), and the midbrain region (including the substantia nigra; C). Untreated (gene on) CaM_α-syn mice (males, n = 1; females, n = 5) and controls (males, n = 2; females, n = 6), treated (gene off) CaM_α-syn mice (males, n = 2; females, n = 2) and equally treated controls (males, n = 3; females, n = 2) were used for HPLC analysis. CaM_α-syn mice showing significant reduction of DA in the olfactory bulb, which was reserved by treatment with dox. Data are presented as mean ± SEM. *p < 0.05, **p < 0.01, compared with corresponding group.
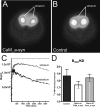
Figure 7.
Striatal DAT binding potential quantification in conditional mice. A, B, Representative transverse color-coded microPET images (of all frames over 60 min acquisition time) of striata were conducted with [11C]
d
-threo-methylphenidate of a CaM_α-syn (A) and the respective control mouse (B). C, Time–activity curve of striatum and cerebellum of a CaM_α-syn mouse were normalized to the injected dose. TACs from striatum and cerebellum were well separated, indicating that the injected tracer did not cause saturation effects or unspecific binding. D, Quantitative analysis of DAT binding potential in the striatum of CaM_α-syn mice (n = 5), respective controls (n = 5), and dox-treated CaM_α-syn mice (n = 3; gene off) revealed reduced DAT binding without reaching statistical significance in CaM_α-syn mice. Error bars indicate SEM.
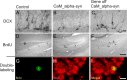
Figure 8.
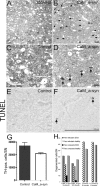
Reversed hippocampal decrease in adult neurogenesis of conditional CaM_α-syn mice. For studies of neurogenesis, 24-week-old CaM_α-syn mice, littermate controls, and treated CaM_α-syn mice were analyzed. Newly generated DCX-labeled neuroblasts were quantified in the hippocampal dentate gyrus (A–C). A significant decrease in DCX-expressing profiles was present in the CaM_α-syn group (B) compared with the control group (A), and this effect was not detectable in mice with ceased transgene expression (C). A reduction of BrdU-labeled, and therefore newly generated, cells (arrows) was present in CaM_α-syn mice (E) in relation to the control group (D) and was not observed in treated (gene off) CaM_α-syn mice (F). Using colocalization of BrdU and NeuN, there was no significant difference in the percentage of neuronal differentiation of BrdU-positive cells among controls, CaM_α-syn mice, and treated (gene off) CaM_α-syn mice, exemplarily shown in G–I: Separate confocal analysis of the fluophores green (488 nm; BrdU; G), red (568 nm; NeuN; H), and merged in the identical focal plane (I). BrdU, Bromodeoxyuridine. Scale bars: A–C, 50 μm; D–F, 400 μm; G–I, 20 μm.
Figure 9.
Motor dysfunction affected motor skill learning and cognitive impairment in CaM_α-syn mice. A, To test motor coordination, the time of CaM_α-syn (n = 8) and respective age- and sex-matched α-syn single-tg control mice (n = 8) to stay on an accelerated rotarod was measured in sessions, consisting of two trials for 5 d, every 6 weeks. Accelerated rotarod documented impaired motor performance in CaM_α-syn mice (open circles) compared with littermate controls (filled circles). Progressive motor impairment started at the age of 30 weeks documented by linear regression, which revealed decreasing time endurance on the rotarod of −13.4 s of the following four sessions (mean slope), which was significantly worse compared with the performance of the control group. Doxycycline treatment to cease expression of the human α-syn is indicated by arrow (gene off). Linear regression analysis revealed halting of progressive motor impairment after cessation of the transgene in symptomatic 58-week-old tg mice, indicated by amelioration of calculated slope to −4.2 s per session. No significant difference in body weight was observed between the CaM_α-syn mice and control group. B, To assess motor skill learning, the difference in time endurance of balancing on the accelerated rotarod between trial 1 and trial 2 of the first tested day in session 1 of 18-week-old CaM_α-syn and control mice was analyzed. Significant improvement was documented only in control mice, revealing impaired motor skill learning in CaM_α-syn mice. C, MWM paradigm: CaM_α-syn tg mice (n = 4) and control mice (n = 4) first received 5 d of cued training (2 trials per day), in which they learned locating a hidden platform to escape from the water. Then, spatial learning was assessed in one probe trial in which the platform is not present. Retention was tested 7 d after the initial learning period (2 trials). To exclude vision impairment, a cued water maze paradigm was performed for 2 d. Working memory was assessed by switching the platform location. D, No significant difference in learning session was found between the two groups. E, After a 7 d retention, CaM_α-syn tg mice demonstrated impaired function of long-term memory by spending significantly more time in the water to find the hidden platform compared with the control group. Error bars indicate SEM. *p < 0.05.
Similar articles
- Synapsin III deficiency hampers α-synuclein aggregation, striatal synaptic damage and nigral cell loss in an AAV-based mouse model of Parkinson's disease.
Faustini G, Longhena F, Varanita T, Bubacco L, Pizzi M, Missale C, Benfenati F, Björklund A, Spano P, Bellucci A. Faustini G, et al. Acta Neuropathol. 2018 Oct;136(4):621-639. doi: 10.1007/s00401-018-1892-1. Epub 2018 Jul 25. Acta Neuropathol. 2018. PMID: 30046897 - Parkin depletion delays motor decline dose-dependently without overtly affecting neuropathology in α-synuclein transgenic mice.
Fournier M, Roux A, Garrigue J, Muriel MP, Blanche P, Lashuel HA, Anderson JP, Barbour R, Huang J, du Montcel ST, Brice A, Corti O. Fournier M, et al. BMC Neurosci. 2013 Nov 5;14:135. doi: 10.1186/1471-2202-14-135. BMC Neurosci. 2013. PMID: 24192137 Free PMC article. - DNA damage preceding dopamine neuron degeneration in A53T human α-synuclein transgenic mice.
Wang D, Yu T, Liu Y, Yan J, Guo Y, Jing Y, Yang X, Song Y, Tian Y. Wang D, et al. Biochem Biophys Res Commun. 2016 Dec 2;481(1-2):104-110. doi: 10.1016/j.bbrc.2016.11.008. Epub 2016 Nov 3. Biochem Biophys Res Commun. 2016. PMID: 27818201 - Evidence for dopaminergic axonal degeneration as an early pathological process in Parkinson's disease.
O'Keeffe GW, Sullivan AM. O'Keeffe GW, et al. Parkinsonism Relat Disord. 2018 Nov;56:9-15. doi: 10.1016/j.parkreldis.2018.06.025. Epub 2018 Jun 19. Parkinsonism Relat Disord. 2018. PMID: 29934196 Review.
Cited by
- Transgenic overexpression of the alpha-synuclein interacting protein synphilin-1 leads to behavioral and neuropathological alterations in mice.
Nuber S, Franck T, Wolburg H, Schumann U, Casadei N, Fischer K, Calaminus C, Pichler BJ, Chanarat S, Teismann P, Schulz JB, Luft AR, Tomiuk J, Wilbertz J, Bornemann A, Krüger R, Riess O. Nuber S, et al. Neurogenetics. 2010 Feb;11(1):107-20. doi: 10.1007/s10048-009-0212-2. Neurogenetics. 2010. PMID: 19760259 - Abrogating Native α-Synuclein Tetramers in Mice Causes a L-DOPA-Responsive Motor Syndrome Closely Resembling Parkinson's Disease.
Nuber S, Rajsombath M, Minakaki G, Winkler J, Müller CP, Ericsson M, Caldarone B, Dettmer U, Selkoe DJ. Nuber S, et al. Neuron. 2018 Oct 10;100(1):75-90.e5. doi: 10.1016/j.neuron.2018.09.014. Neuron. 2018. PMID: 30308173 Free PMC article. - A β-synuclein mutation linked to dementia produces neurodegeneration when expressed in mouse brain.
Fujita M, Sugama S, Sekiyama K, Sekigawa A, Tsukui T, Nakai M, Waragai M, Takenouchi T, Takamatsu Y, Wei J, Rockenstein E, Laspada AR, Masliah E, Inoue S, Hashimoto M. Fujita M, et al. Nat Commun. 2010 Nov 2;1:110. doi: 10.1038/ncomms1101. Nat Commun. 2010. PMID: 21045828 Free PMC article. - Value of genetic models in understanding the cause and mechanisms of Parkinson's disease.
Moore DJ, Dawson TM. Moore DJ, et al. Curr Neurol Neurosci Rep. 2008 Jul;8(4):288-96. doi: 10.1007/s11910-008-0045-7. Curr Neurol Neurosci Rep. 2008. PMID: 18590612 Free PMC article. Review. - The role of adult hippocampal neurogenesis in brain health and disease.
Toda T, Parylak SL, Linker SB, Gage FH. Toda T, et al. Mol Psychiatry. 2019 Jan;24(1):67-87. doi: 10.1038/s41380-018-0036-2. Epub 2018 Apr 20. Mol Psychiatry. 2019. PMID: 29679070 Free PMC article. Review.
References
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. - PubMed
- Antonini A, Moresco RM, Gobbo C, De Notaris R, Panzacchi A, Barone P, Calzetti S, Negrotti A, Pezzoli G, Fazio F. The status of dopamine nerve terminals in Parkinson's disease and essential tremor: a PET study with the tracer [11-C]FE-CIT. Neurol Sci. 2001;22:47–48. - PubMed
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. - PubMed
- Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973;20:415–455. - PubMed
- Bezard E, Gross CE. Compensatory mechanisms in experimental and human parkinsonism: towards a dynamic approach. Prog Neurobiol. 1998;55:93–116. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous