Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons - PubMed (original) (raw)
Comparative Study
Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons
Matthew R Hodges et al. J Neurosci. 2008.
Abstract
Serotonergic neurons project widely throughout the CNS and modulate many different brain functions. Particularly important, but controversial, are the contributions of serotonin (5-HT) neurons to respiratory and thermoregulatory control. To better define the roles of 5-HT neurons in breathing and thermoregulation, we took advantage of a unique conditional knock-out mouse in which Lmx1b is genetically deleted in Pet1-expressing cells (Lmx1b(f/f/p)), resulting in near-complete absence of central 5-HT neurons. Here, we show that the hypercapnic ventilatory response in adult Lmx1b(f/f/p) mice was decreased by 50% compared with wild-type mice, whereas baseline ventilation and the hypoxic ventilatory response were normal. In addition, Lmx1b(f/f/p) mice rapidly became hypothermic when exposed to an ambient temperature of 4 degrees C, decreasing core temperature to 30 degrees C within 120 min. This failure of thermoregulation was caused by impaired shivering and nonshivering thermogenesis, whereas thermosensory perception and heat conservation were normal. Finally, intracerebroventricular infusion of 5-HT stimulated baseline ventilation, and rescued the blunted hypercapnic ventilatory response. These data identify a previously unrecognized role of 5-HT neurons in the CO(2) chemoreflex, whereby they enhance the response of the rest of the respiratory network to CO(2). We conclude that the proper function of the 5-HT system is particularly important under conditions of environmental stress and contributes significantly to the hypercapnic ventilatory response and thermoregulatory cold defense.
Figures
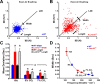
Figure 1.
Breath-to-breath variability in WT and Lmx1bf/f/p mice. A, B, Poincaré plot of the IBI of sequential breaths during baseline normoxic ventilation in WT (A; n = 7) and Lmx1bf/f/p (B; n = 7) mice. C, Mean width (short-term variation) and length (long-term variation) in room air (RA), 5% and 7% CO2. D, Relationship of scatter plot width and breathing frequency in WT mice under varying conditions. Note that Lmx1bf/f/p data fall along the line fit to the WT data (variability = 14,660 × frequency−2.4754), indicating the variation in IBI was caused by differences in breathing frequency rather than breathing instability. Unpaired t test was used; *p < 0.05 for WT versus Lmx1bf/f/p; #p < 0.05 for RA versus 5% or 7% CO2. Data are mean ± SEM.
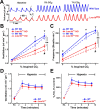
Figure 2.
Lmx1bf/f/p mice have decreased ventilatory drive and their hypercapnic ventilatory response is severely blunted. A, Plethysmography recordings from WT (blue) and Lmx1bf/f/p (red) mice at rest, and while breathing 5% and 7% CO2 in normoxia (21% O2). Dashed lines highlight decreased slope of the inspiratory phase (ventilatory drive), and vertical lines mark the IBI. B, C, Ventilation (B) and breathing frequency (C) during hypercapnia under normoxic (NO) and hyperoxic (HO; 50% O2) conditions (n = 10 WT NO, 8 Lmx1bf/f/p NO, 11 WT HO, 8 Lmx1bf/f/p HO). D, E, Ventilation (D) and convection requirement (E; VE/_V_O2; expressed as a percentage of control) during exposure to room air (RA) and hypoxia (n = 8 WT, 8 Lmx1bf/f/p). For two-way ANOVA (genotype and condition as factors), **p < 0.05. For unpaired t test, *p < 0.05 (WT versus Lmx1bf/f/p) for both normoxia and hyperoxia. For paired t test, #p < 0.05 for normoxia versus hyperoxia in Lmx1bf/f/p. Data are mean ± SEM.
Figure 3.
Lmx1bf/f/p mice fail to maintain their body temperature during cold exposure. A, mean core body temperature (_T_Core; top) and activity [arbitrary units (A.U.); bottom] over 24 h in WT (blue) and Lmx1bf/f/p (red) mice at 24°C. B, Mean _T_Core and activity by phase [light (L); dark (D)] at 24°C (n = 6 WT, 5 Lmx1bf/f/p) and 30°C (n = 6 WT, 6 Lmx1bf/f/p). C–F, Core temperatures (T_Core) in individual WT (n = 5; blue) and Lmx1bf/f/p (n = 7; red) mice before, during and after exposure to 4°C (C, D) or 12°C (E, F). Lines in D and F are discontinuous to align recovery period, and arrows in F indicate removal of animals from the cold caused by marked hypothermia. Unpaired t test was used; *p < 0.05 for WT versus Lmx1bf/f/p; #p < 0.05 for light versus dark; †_p < 0.05 for 24°C versus 30°C. Data are mean ± SEM.
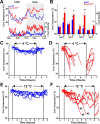
Figure 4.
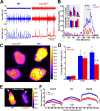
Lmx1bf/f/p mice have normal thermosensory perception and activate heat conservation mechanisms normally in response to cold. A, Visible light (top) and infrared (bottom) images of thermocline. B, Summary data from thermocline experiments (n = 5 WT, 6 Lmx1bf/f/p). Note that the mean temperature that mice selected over time (top inset) and overall (bottom inset) were not different between genotypes. C, Thermographic images of tail (arrows) surface temperatures in WT (left) and Lmx1bf/f/p (right) mice. D, Summary data of tail surface temperature versus ambient temperature when mice were exposed to cold (n = 10 WT, 8 Lmx1bf/f/p). Data are mean ± SEM.
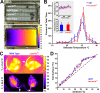
Figure 5.
Shivering and brown adipose tissue activation are both impaired in Lmx1bf/f/p mice. A, Recordings of nuchal muscle activity (top two traces, raw data; bottom trace, RMS) in warm (top trace; 28°C) and cold (bottom two traces; 4°C) _T_Amb. Note that regular shivering was induced by cold exposure in the WT mouse (left; blue), but bursts were less frequent in the Lmx1bf/f/p mouse (right; red). B, Group means (n = 4 WT, blue; 4 Lmx1bf/f/p, red) of burst frequency before, during and after cold exposure. Insets, Total nuchal muscle activity (top; expressed as absolute and Δ total EMG), and burst duration (bottom) 1 h before [warm (W)] and during the last 30 min of cold exposure [cold (C)]. C, Thermographic images of the shaved back in WT (right) and Lmx1bf/f/p (left) mice during cold exposure. D, Summary data of surface temperature differences between the IBAT region (solid circles in C) and the lower back (dashed circles in C) at _T_Amb of 35, 10, and 7°C. Note that there was significant IBAT activation in both WT (n = 8) and Lmx1bf/f/p (n = 8) mice at 10 and 7°C, but the activation was less in Lmx1bf/f/p mice at 7°C. E, Thermographic image of neonatal (P5) WT and Lmx1bf/f/p mice at T_Amb of 20°C. Arrows denote the head. F, Line profile summary data of back surface temperatures at three ages (P2–P3, P4, and P5–P6). Note the visible yellow (warm) band in the P5 WT mouse, indicating IBAT activation. Two-way ANOVA (genotype and time, or ambient temperature, and tail surface temperature as factors) or unpaired t test was used; †_p < 0.05 for warm versus cold; *p < 0.05 for WT versus Lmx1bf/f/p. Data are mean ± SEM.
Figure 6.
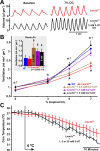
Exogenous 5-HT rescues the hypercapnic ventilatory response. A, Plethysmography recordings from an Lmx1bf/f/p mouse breathing 0% CO2 and 7% CO2 (both in 50% O2) before (red), and on the second day of intracerebroventricular infusion of 5 m
m
5-HT (black). B, Effect of CO2 on ventilation in WT mice (blue; n = 7), Lmx1bf/f/p mice before intracerebroventricular infusion (red; n = 9), and Lmx1bf/f/p mice on the second day of intracerebroventricular infusion of 5-HT at concentrations (within the pump) of 0.5 (brown; n = 5), 1.0 (purple; n = 7), and 5.0 m
m
(black; n = 6). Data were obtained in 50% O2. Inset, Baseline ventilation in 21% O2. C, Mean _T_Core in Lmx1bf/f/p mice with (black) or without (red) intracerebroventricular 5-HT infusion. Data were combined from all animals at 5-HT concentrations of 1, 5, and 25 m
m
. Two-way ANOVA (p < 0.001 for 5-HT level and condition), and unpaired t test were used; $p < 0.05 for intracerebroventricular 5-HT versus control; *p < 0.05 for 1.0 m
m
5-HT versus control; †p < 0.05 for 5.0 m
m
5-HT versus control; #p < 0.05 for Lmx1bf/f/p control and 0.5 m
m
5-HT versus WT. Data are mean ± SEM.
Similar articles
- Interaction between defects in ventilatory and thermoregulatory control in mice lacking 5-HT neurons.
Hodges MR, Richerson GB. Hodges MR, et al. Respir Physiol Neurobiol. 2008 Dec 31;164(3):350-7. doi: 10.1016/j.resp.2008.08.003. Epub 2008 Aug 15. Respir Physiol Neurobiol. 2008. PMID: 18775520 Free PMC article. - Diphtheria toxin treatment of Pet-1-Cre floxed diphtheria toxin receptor mice disrupts thermoregulation without affecting respiratory chemoreception.
Cerpa V, Gonzalez A, Richerson GB. Cerpa V, et al. Neuroscience. 2014 Oct 24;279:65-76. doi: 10.1016/j.neuroscience.2014.08.018. Epub 2014 Aug 27. Neuroscience. 2014. PMID: 25171790 Free PMC article. - Altered ventilatory and thermoregulatory control in male and female adult Pet-1 null mice.
Hodges MR, Best S, Richerson GB. Hodges MR, et al. Respir Physiol Neurobiol. 2011 Jul 31;177(2):133-40. doi: 10.1016/j.resp.2011.03.020. Epub 2011 Mar 29. Respir Physiol Neurobiol. 2011. PMID: 21453797 Free PMC article. - The role of medullary serotonin (5-HT) neurons in respiratory control: contributions to eupneic ventilation, CO2 chemoreception, and thermoregulation.
Hodges MR, Richerson GB. Hodges MR, et al. J Appl Physiol (1985). 2010 May;108(5):1425-32. doi: 10.1152/japplphysiol.01270.2009. Epub 2010 Feb 4. J Appl Physiol (1985). 2010. PMID: 20133432 Free PMC article. Review. - Serotonin neurons and central respiratory chemoreception: where are we now?
Teran FA, Massey CA, Richerson GB. Teran FA, et al. Prog Brain Res. 2014;209:207-33. doi: 10.1016/B978-0-444-63274-6.00011-4. Prog Brain Res. 2014. PMID: 24746050 Free PMC article. Review.
Cited by
- Breathing disturbances in Rett syndrome.
Ramirez JM, Karlen-Amarante M, Wang JJ, Huff A, Burgraff N. Ramirez JM, et al. Handb Clin Neurol. 2022;189:139-151. doi: 10.1016/B978-0-323-91532-8.00018-5. Handb Clin Neurol. 2022. PMID: 36031301 Free PMC article. Review. - Insomnia Caused by Serotonin Depletion is Due to Hypothermia.
Murray NM, Buchanan GF, Richerson GB. Murray NM, et al. Sleep. 2015 Dec 1;38(12):1985-93. doi: 10.5665/sleep.5256. Sleep. 2015. PMID: 26194567 Free PMC article. - The neurobiology of sensing respiratory gases for the control of animal behavior.
Ma DK, Ringstad N. Ma DK, et al. Front Biol (Beijing). 2012 Jun;7(3):246-253. doi: 10.1007/s11515-012-1219-x. Front Biol (Beijing). 2012. PMID: 22876258 Free PMC article. - Peripheral-central chemoreceptor interaction and the significance of a critical period in the development of respiratory control.
Wong-Riley MT, Liu Q, Gao XP. Wong-Riley MT, et al. Respir Physiol Neurobiol. 2013 Jan 1;185(1):156-69. doi: 10.1016/j.resp.2012.05.026. Epub 2012 Jun 8. Respir Physiol Neurobiol. 2013. PMID: 22684042 Free PMC article. Review. - Introduction. Human thermoregulatory research.
Shido O, Watanabe T, Taylor NA, Havenith G. Shido O, et al. Eur J Appl Physiol. 2010 May;109(1):1-3. doi: 10.1007/s00421-010-1425-7. Epub 2010 Mar 10. Eur J Appl Physiol. 2010. PMID: 20217114 No abstract available.
References
- Annerbrink K, Olsson M, Melchior LK, Hedner J, Eriksson E. Serotonin depletion increases respiratory variability in freely moving rats: implications for panic disorder. Int J Neuropsychopharmacol. 2003;6:51–56. - PubMed
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci. 2004;7:48–55. - PubMed
- Boulant JA. Role of the preoptic-anterior hypothalamus in thermoregulation and fever. Clin Infect Dis. 2000;31(Suppl 5):S157–S161. - PubMed
- Boulant JA. Neuronal basis of Hammel's model for set-point thermoregulation. J Appl Physiol. 2006;100:1347–1354. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS046036/NS/NINDS NIH HHS/United States
- P01HD36379/HD/NICHD NIH HHS/United States
- R01 MH062723/MH/NIMH NIH HHS/United States
- HD052772/HD/NICHD NIH HHS/United States
- P01 HD036379/HD/NICHD NIH HHS/United States
- R01 HD052772/HD/NICHD NIH HHS/United States
- R01MH62723/MH/NIMH NIH HHS/United States
- U54 NS041069/NS/NINDS NIH HHS/United States
- 5 R01NS046036-04/NS/NINDS NIH HHS/United States
- 5 U54 NS41069-06A1/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases