Induction of neurogenesis in nonconventional neurogenic regions of the adult central nervous system by niche astrocyte-produced signals - PubMed (original) (raw)
Induction of neurogenesis in nonconventional neurogenic regions of the adult central nervous system by niche astrocyte-produced signals
Jianwei Jiao et al. Stem Cells. 2008 May.
Abstract
The central nervous system (CNS) of adult mammals regenerates poorly; in vivo, neurogenesis occurs only in two restricted areas, the hippocampal subgranular zone (SGZ) and the subventricular zone (SVZ). Neurogenic potential depends on both the intrinsic properties of neural progenitors and the environment, or niche, in which progenitor cells reside. Isolation of multipotent progenitor cells from broad CNS regions suggests that the neurogenic potential of the adult CNS is dictated by local environmental cues. Here, we report that astrocytes in the neurogenic brain regions, the SGZ and SVZ, of adult mice release molecular signals, such as sonic hedgehog (Shh), that stimulate adult neural progenitors to reenter the cell cycle and generate new neurons in vitro and in vivo. Transplantation of SGZ astrocytes or application of Shh caused de novo neurogenesis from the non-neurogenic neocortex of adult mice. These findings identify a molecular target that can activate the dormant neurogenic potential from nonconventional neurogenic regions of the adult CNS and suggest a novel mechanism of neural replacement therapy for treating neurodegenerative disease and injury without transplanting exogenous cells.
Figures
Figure 1
Development of neurospheres in adult cortical cell cultures. (A): Schematic illustration of adult cortical cells that were cocultured with feeder cells. (B): Niche astrocytes induced neurosphere formation from adult cortical cells. Large floating neurospheres developed after 8 days of incubation in cortical cell cultures prepared from adult mice that were plated in the same well with A-SGZ or AST-P0 but not in those that were cocultured with NIH3T3. Neurospheres were stained positive for antinestin. Scale bar = 40 µm. (C): Numbers of neurospheres developed in dissociated adult cortical cell cultures plated in the presence or absence of various feeder cells after an 8-day incubation. Neurospheres larger than 50 cells were counted (*, p < .001; Student’s t test and analysis of variance). (D): A green fluorescent protein+ neurosphere developed in astrocyte-cortical cell cocultures, in which cortical cells were derived from a GFPtg mouse. Scale bar = 25 µm. (E): Number of neurospheres developed in cortical cell cultures in the presence of control medium (control) or N-CM or A-CM culture. Abbreviations: A-CM, P0 subgranular zone astrocyte; A-Cx, adult cortical cells; A-SC, adult spinal cord astrocytes; A-SGZ, adult subgranular zone; AST-P0, P0 brain astrocytes; N-CM, medium conditioned by NIH3T3; NIH3T3, NIH3T3 fibroblasts.
Figure 2
Wide distribution of neural progenitors in the adult central nervous system. (A): Number of neurospheres developed in cultures derived from gray-matter cells of the cerebellum and spinal cord of adult mice that were cultured in the absence (control) or presence of P0 Astro for 8 days. Results represent the mean ± SD (n = 9; * p < .01; Student’s t test). (B): Sphere-forming cells undergo active cell division. Sphere-derived cells were dissociated and cultured in the presence of BrdU for 24 hours and were labeled by anti-BrdU (red) and nuclei (DAPI; blue) staining. Scale bar = 40 µm. Abbreviations: Astro, astrocytes; BrdU, 5′-bromo-2′-deoxyuridine; DAPI, 4′,6-diamidino-2-phenylindole dihydrochloride.
Figure 3
Neural progenitor cells display multipotency in vitro. (A): Sphere-derived cells differentiated into neurons, astrocytes, and oligodendrocytes in culture. Neurospheres isolated from cortical cell cultures were dissociated, plated on Matrigel-coated chamber slides, and cultured in N2 medium containing retinoic acid and 2% fetal bovine serum. After a 7-day incubation, cultures were fixed and stained with antibodies specific for Tuj1, MAP-2, S100, and O4. Overlay image shows a Tuj1+ (green) neuron coexpressing the mature neuronal marker MAP2 (red) and DAPI (blue). Scale bar = 40 µm. (B, C): Quantification of cell differentiation in dissociated neurosphere cell cultures, of which the neurospheres were isolated either from the neocortex-, cerebellum-, and spinal cord-niche astrocyte cocultures (B) or from cortical cell cultures in the absence of niche astrocytes (C). Data represent percentage of cells immunostained for neuronal (Tuj1-positive), astrocyte (S100), and oligodendrocyte (O4) markers over total number of cells in culture ± SD. Note that in the absence of niche astrocytes, neurosphere cells derived from the neocortex exhibited lower neurogenic potential but preferentially differentiated into astroglia compared with those cells derived from niche astrocyte cocultures. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole dihydrochloride; MAP2, microtubule-associated protein 2; Tuj1, β-III-tubulin.
Figure 4
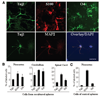
Sphere-forming cells are originated from GFAP-expressing cells. (A): Sphere-forming cells are GFAP+ and nestin+. Results of reverse transcription-polymerase chain reaction show that NS were GFAP+ and nestin+, whereas primary Cx expressed GFAP and S100. (B): Fluorescence confocal images of single optical slices were taken from adult cortical sections double-labeled by primary antibodies specific for GFAP and nestin, identifying a subpopulation of GFAP+ (red)/nestin+ (green) cells. Arrows point to cells positively labeled by both anti-GFAP and anti-nestin. Scale bars = 30 µm. (C): A GFP+ neurosphere formed in cocultures using GFP+ Cx sorted out from GFAP-GFP mice. Note that because GFP expression is driven under the GFAP promoter, only GFAP+ cells from these mice were GFP+. Scale bar = 20 µm. (D): Number of neurospheres developed from unsorted (Cx) and sorted GFP+ (GFAP+) and GFP− (GFAP−) cortical cell cultures in the absence ((−) astro) or presence ((+) astro) of P0 astro. Results represent the mean ± SD (n = 6; *, p < .001; Student’s t test and analysis of variance). Abbreviations: Astro, astrocytes; Cx, cortical cells; GFAP, glial fibrillary acidic protein; GFP, green fluorescent protein; NS, neurosphere cells.
Figure 5
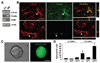
Depletion of GFAP-expressing cells abolishes neurosphere formation in cortical-astrocyte cocultures. (A): TK+ cells in GFAP-TK mice are GFAP+. Confocal photomicrographs of single optical slices taken from the cortex of adult GFAP-TK mice were double-labeled by anti-TK (green) and anti-GFAP (red). Scale bars = 40 µm. (B): Number of neurospheres counted from cortical-astrocyte cocultures, in which cortical cells were derived from GFAP-TK or WT mice, in the absence or presence of GCV. Note that GCV does not affect the number of neurospheres in WT cultures. Results represent the mean ± SD (n = 6; *, p < .01; Student’s t test). (C): Confocal photomicrographs of NG2 immunolabeling showing that the GFAP+ neurosphere was NG2−. GFAP+ neurospheres were derived from dissociated neocortical cell cultures, and NG2+ neurospheres were collected from cultured NG2+ cells sorted out from the neocortex by fluorescence-activated cell sorting using anti-A2B5. Scale bars = 40 µm. (D): Results of reverse transcription-polymerase chain reaction reveal low levels of NG2 and A2B5 mRNAs in GFAP+ neurosphere cells compared with NG2+ neurospheres. (E, F): Quantification of neural and glial differentiation in cultures derived from secondary or tertiary GFAP+ and NG2+ spheres isolated from the neocortex or WT (E) or from GFAP-TK (F) mice in the absence (E) or presence (F) of GCV. Note the reduction of astrocyte, but not neuron or oligodendrocyte, differentiation in NG2+ cell cultures derived from GFAP-TK mice after GCV treatment (F), indicating the selective effect of GCV on GFAP+ cells. Data represent percentage of cells immunostained for Tuj1, S100, and O4 over total number of cells in culture ± SD. Abbreviations: GCV, ganciclovir; GFAP, glial fibrillary acidic protein; immuno+, immunopositive; TK, thymidine kinase; Tuj1, β-III-tubulin; WT, wild-type.
Figure 6
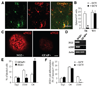
Transplantation of niche Astro induces progenitor cell proliferation and neurogenesis in the adult neocortex. (A): Schematic illustration of a sagittal section of a mouse brain and a photomicrograph of an overlay image of anti-BrdU (red) and green fluorescent protein (GFP) immunofluorescence in a cortical section of a mouse that received a control injection (cont). Very few BrdU+ cells were observed in the control mouse brain. Quantification of BrdU+ cells in brain sections taken from mice treated with Cont or niche Astro. (B): Numerous BrdU+ cells were detected in cortical areas surrounding GFP+ Astro transplants (green). Two weeks after receiving niche Astro transplants, mouse brain sections were collected and immunolabeled for BrdU (red). Arrowheads indicate BrdU+ nuclei. Note that BrdU+ cells appeared adjacent to the Astro implant. Scale bar = 40 µm. (C): Confocal photomicrographs of single optic slices taken from the vicinity of Astro-injected cortical areas showing newly born, BrdU+ cells (red) colocalized with immunolabeling of neuronal markers NeuN (green), Dcx (blue), and HuD (blue). Scale bars = 40 µm (A, B) and 20 µm (C). Abbreviations: Astro, astrocytes; BrdU, 5′-bromo-2′-deoxyuridine; Cont, control injection; Dcx, doublecortin; SGZ, subgranular zone; SVZ, subventricular zone.
Figure 7
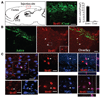
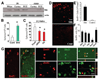
Shh as a component of niche astrocyte-produced neurogenic niche. (A): Shh expression by niche astrocytes or neurogenic brain regions of adult mice. Representative Western blot analyses of triplicate experiments showing Shh expression in P0Ast, the cerebral cortex and SGZ of P0 and adult mice, but not in the non-neurogenic cortex of adult mice. (B): Shh is a positive regulator for neural progenitor cell proliferation in culture. Quantification of neurosphere formation in dissociated cortical cell cultures in the absence (Cont) or presence of Shh (50 ng/ml). (C): αShh (1 µg/ml) and its inhibitor CP (5 µM) diminish neurosphere formation in cortical-astrocyte ((+)Astro) cocultures. Results represent the mean value ± SD (n = 5; *, p < .01; Student’s t test). (D): Administration of Shh induced cell proliferation in the adult mouse neocortex in vivo. A solution containing soluble Shh or phosphate-buffered saline (PBS) (as Cont) was administered to the neocortex of adult mice. Two weeks postinjection, numerous BrdU+ cells (red) were observed in the cortical area surrounding the Shh injection site (Shh) but not the PBS injection site (Cont). (E, F): Quantification of BrdU+ cells (E) and BrdU+ cells that were double-labeled for another cell marker (HuD, DCX, Tuj1, NeuN, or GFAP) (F) in brain sections taken from mice received PBS (white bar) or Shh (50 ng/ml) (black bar) injection. (G): Shh-induced BrdU+ cells differentiate into neurons in vivo. Confocal photomicrographs of single optical slices taken form the cortex of adult of mice received Shh injection showing subsets of BrdU+ precursors are positive for neuronal marker HuD, NeuN, and βIII-tubulin (Tuj1). Scale bars = 50 µm (D) and 30 µm (E). Abbreviations: BrdU, 5′-bromo-2′-deoxyuridine; Cont, control; CP, cyclopamine; DCX, doublecortin; GFAP, glial fibrillary acidic protein; P0Ast, P0 brain astrocytes; SGZ, subgranular zone; Shh, sonic hedgehog; αShh, sonic hedgehog neutralizing antibody; Tuj1, β-III-tubulin.
Similar articles
- A role for DNA-dependent activator of interferon regulatory factor in the recognition of herpes simplex virus type 1 by glial cells.
Furr SR, Chauhan VS, Moerdyk-Schauwecker MJ, Marriott I. Furr SR, et al. J Neuroinflammation. 2011 Aug 12;8:99. doi: 10.1186/1742-2094-8-99. J Neuroinflammation. 2011. PMID: 21838860 Free PMC article. - Qualitative evidence synthesis informing our understanding of people's perceptions and experiences of targeted digital communication.
Ryan R, Hill S. Ryan R, et al. Cochrane Database Syst Rev. 2019 Oct 23;10(10):ED000141. doi: 10.1002/14651858.ED000141. Cochrane Database Syst Rev. 2019. PMID: 31643081 Free PMC article. - Enabling Systemic Identification and Functionality Profiling for Cdc42 Homeostatic Modulators.
Malasala S, Azimian F, Chen YH, Twiss JL, Boykin C, Akhtar SN, Lu Q. Malasala S, et al. bioRxiv [Preprint]. 2024 Jan 8:2024.01.05.574351. doi: 10.1101/2024.01.05.574351. bioRxiv. 2024. PMID: 38260445 Free PMC article. Updated. Preprint. - Valley fever under a changing climate in the United States.
Howard MH, Sayes CM, Giesy JP, Li Y. Howard MH, et al. Environ Int. 2024 Nov;193:109066. doi: 10.1016/j.envint.2024.109066. Epub 2024 Oct 11. Environ Int. 2024. PMID: 39432997 Review. - Harnessing Biological Insight to Accelerate Tuberculosis Drug Discovery.
de Wet TJ, Warner DF, Mizrahi V. de Wet TJ, et al. Acc Chem Res. 2019 Aug 20;52(8):2340-2348. doi: 10.1021/acs.accounts.9b00275. Epub 2019 Jul 30. Acc Chem Res. 2019. PMID: 31361123 Free PMC article. Review.
Cited by
- Bone Marrow Derived Mesenchymal Stem Cells in Addiction Related Hippocampal Damages.
Rafaiee R, Ahmadiankia N. Rafaiee R, et al. Int J Mol Cell Med. 2018 Spring;7(2):69-79. doi: 10.22088/IJMCM.BUMS.7.2.69. Epub 2018 Jun 20. Int J Mol Cell Med. 2018. PMID: 30276162 Free PMC article. Review. - Mobilizing endogenous stem cells for retinal repair.
Yu H, Vu TH, Cho KS, Guo C, Chen DF. Yu H, et al. Transl Res. 2014 Apr;163(4):387-98. doi: 10.1016/j.trsl.2013.11.011. Epub 2013 Nov 22. Transl Res. 2014. PMID: 24333552 Free PMC article. Review. - Sonic hedgehog pathway activation is induced by acute brain injury and regulated by injury-related inflammation.
Amankulor NM, Hambardzumyan D, Pyonteck SM, Becher OJ, Joyce JA, Holland EC. Amankulor NM, et al. J Neurosci. 2009 Aug 19;29(33):10299-308. doi: 10.1523/JNEUROSCI.2500-09.2009. J Neurosci. 2009. PMID: 19692604 Free PMC article. - Adult Neurogenesis in the Subventricular Zone and Its Regulation After Ischemic Stroke: Implications for Therapeutic Approaches.
Dillen Y, Kemps H, Gervois P, Wolfs E, Bronckaers A. Dillen Y, et al. Transl Stroke Res. 2020 Feb;11(1):60-79. doi: 10.1007/s12975-019-00717-8. Epub 2019 Jul 15. Transl Stroke Res. 2020. PMID: 31309427 Review. - The BAF45D Protein Is Preferentially Expressed in Adult Neurogenic Zones and in Neurons and May Be Required for Retinoid Acid Induced PAX6 Expression.
Liu C, Sun R, Huang J, Zhang D, Huang D, Qi W, Wang S, Xie F, Shen Y, Shen C. Liu C, et al. Front Neuroanat. 2017 Nov 6;11:94. doi: 10.3389/fnana.2017.00094. eCollection 2017. Front Neuroanat. 2017. PMID: 29163067 Free PMC article.
References
- Lie DC, Song H, Colamarino SA, et al. Neurogenesis in the adult brain: New strategies for central nervous system diseases. Annu Rev Pharmacol Toxicol. 2004;44:399–421. - PubMed
- Kornack DR, Rakic P. Cell proliferation without neurogenesis in adult primate neocortex. Science. 2001;294:2127–2130. - PubMed
- Kokoeva MV, Yin H, Flier JS. Neurogenesis in the hypothalamus of adult mice: Potential role in energy balance. Science. 2005;310:679–683. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 EY017641-01A2/EY/NEI NIH HHS/United States
- R01 EY012983/EY/NEI NIH HHS/United States
- R01-EY017641/EY/NEI NIH HHS/United States
- P30 EY0 03790/EY/NEI NIH HHS/United States
- R01 EY012983-05S1/EY/NEI NIH HHS/United States
- P30 EY003790/EY/NEI NIH HHS/United States
- R01 EY017641/EY/NEI NIH HHS/United States