A model for neuronal competition during development - PubMed (original) (raw)
A model for neuronal competition during development
Christopher D Deppmann et al. Science. 2008.
Abstract
We report that developmental competition between sympathetic neurons for survival is critically dependent on a sensitization process initiated by target innervation and mediated by a series of feedback loops. Target-derived nerve growth factor (NGF) promoted expression of its own receptor TrkA in mouse and rat neurons and prolonged TrkA-mediated signals. NGF also controlled expression of brain-derived neurotrophic factor and neurotrophin-4, which, through the receptor p75, can kill neighboring neurons with low retrograde NGF-TrkA signaling whereas neurons with high NGF-TrkA signaling are protected. Perturbation of any of these feedback loops disrupts the dynamics of competition. We suggest that three target-initiated events are essential for rapid and robust competition between neurons: sensitization, paracrine apoptotic signaling, and protection from such effects.
Figures
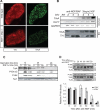
Fig. 1
NGF promotes TrkA expression and the duration of TrkA signaling events. (A) Immunohistochemistry for TrkA and tyrosine hydroxylase (TH) on SCGs from wild-type and _NGF_–/–;_Bax_–/– animals. (B) TrkA, KLF7, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) levels were measured by RT-PCR and TrkA and TH by Western blot (WB) after treatment of cultured rat sympathetic neurons with NGF (30 ng/ml) for indicated times. (C) TrkA, p-ERK, p-Akt, and TuJI measured by WB in P0.5 rat sympathetic neurons that were deprived of NGF for the indicated times followed by reexposure to NGF (30 ng/ml) for 20 or 60 min. (D) Sympathetic neuron cultures from E18 rats were grown in the presence of 30 ng/ml NGF for 1 day in vitro (DIV) or 4 DIVs. NGF-specific antibody (anti-NGF) was added, and NGF signaling events were assessed at the indicated times after NGF deprivation. Graph compares relative p-Akt levels for this experiment. Scale bar in (A), 50 μm. *P < 0.05 analysis of variance (ANOVA) followed by Tukey's post hoc test.
Fig. 2
Requirement for both NGF-dependent TrkA expression and signal duration during competition revealed by computer simulations. Shown are model simulation results for 100 neurons under different competitive conditions. (A to C) Simulated trophic signaling state of 100 individual neurons with indicated parameters over time. (A) TrkA production and signaling duration were held constant. (B) TrkA production was NGF-dependent but signaling duration was held constant. (C) TrkA production and signaling duration were NGF-dependent. (D to F) Comparison of various dynamic elements as a function of time in simulations from (A) to (C). (D) Average trophic signal strength; (E) cell survival; (F) relative amount of NGF at the target. The black, green, and blue lines in (D), (E), and (F) represent results of simulations described for (A), (B), and (C).
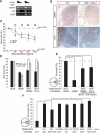
Fig. 3
Antagonism of retrograde NGF survival signaling by NGF induced BDNF and NT4 expression in sympathetic neurons. (A) RT-PCR for BDNF, NT-4, and GAPDH with RNA from NGF-treated (24 hours) or NGF-deprived sympathetic neurons from P0 mice grown 3 DIVs. (B) In situ hybridization for BDNF and NT-4 in wild-type and _NGF_–/–;_Bax_–/– animals at P0. Scale bar, 50 μm. (C to F) Neurotrophin promoted p75-dependent cell death of cultured sympathetic neurons. Indicated neurotrophins were applied for 36 hours, cell survival was determined by Hoechst staining, and results are means ± SEM (n = 4 experiments). (C) Survival of P0 to P2 rat sympathetic neurons maintained in medium containing NGF (1 ng/ml). Green dashed line represents maximum survival, and red dashed line indicates maximum death, both conditions were assessed after 36 hours with either 1 ng/ml of NGF or NGF-specific antibody (anti-NGF), respectively. (D) Survival of P0 to P2 _p75_–/– or wild-type mouse sympathetic neurons treated with NGF (1 ng/ml)or NGF-specific antibody (αNGF). (E and F) P0 sympathetic neurons grown in compartmentalized chambers (represented by illustration left of graphs) for 5 to 7 days before medium was changed to contain NGF (5 ng/ml) on the distal axons. The indicated neurotrophins or NGF-specific antibody (αNGF) was applied to the cell bodies for 36 hours before assessing survival. Unless otherwise indicated, BDNF and NT-4 were applied at a concentration of 250 ng/ml *P < 0.01 ANOVA followed by Tukey's post hoc test.
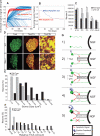
Fig. 4
Mathematical modeling predicts changes in competition dynamics that are corroborated in vivo. (A and B) Simulations of neuronal competition with and without NGF-dependent production of an apoptotic cue (red and blue, respectively). (A) Trophic signaling strength of 100 individual neurons as a function of time. (B) Cell survival as a function of time. (C) Cell counts of Nissl-stained SCG sections from _p75_–/– or wild-type mice at indicated developmental ages. Results are means ± SEM (n = 3 for each age). (D) Immunostaining for TH and TrkA in SCGs from _Bax_–/– and wild-type animals. Scale bar, 50 μm. (Right) Boxed region in middle panels magnified 5×. (E and F) Quantification of soma size (E) and relative TrkA amount (F) in P5 _Bax_–/– versus wild-type SCGs. Results are represented as a percentage of total neurons counted. *P < 0.01 ANOVA followed by Tukey's post hoc test. (G) Model for developmental competition: 1) Before target innervation neurons are modestly responsive to NGF; 2) upon target innervation and exposure to NGF, levels of TrkA, then BDNF and NT-4 are increased; 3) induction of p75 expression, as well as differential sensitization of neurons, by modulation of NGF-TrkA signal strength and duration. 4) BDNF and NT-4 (apoptotic cues) kill neurons with low NGF-TrkA signaling; neurons with high NGF-TrkA signaling are resistant; 5) selection and neuronal death.
Similar articles
- Nerve growth factor survival signaling in cultured hippocampal neurons is mediated through TrkA and requires the common neurotrophin receptor P75.
Culmsee C, Gerling N, Lehmann M, Nikolova-Karakashian M, Prehn JH, Mattson MP, Krieglstein J. Culmsee C, et al. Neuroscience. 2002;115(4):1089-108. doi: 10.1016/s0306-4522(02)00539-0. Neuroscience. 2002. PMID: 12453482 - p53 is essential for developmental neuron death as regulated by the TrkA and p75 neurotrophin receptors.
Aloyz RS, Bamji SX, Pozniak CD, Toma JG, Atwal J, Kaplan DR, Miller FD. Aloyz RS, et al. J Cell Biol. 1998 Dec 14;143(6):1691-703. doi: 10.1083/jcb.143.6.1691. J Cell Biol. 1998. PMID: 9852160 Free PMC article. - A nerve growth factor-induced retrograde survival signal mediated by mechanisms downstream of TrkA.
Mok SA, Campenot RB. Mok SA, et al. Neuropharmacology. 2007 Feb;52(2):270-8. doi: 10.1016/j.neuropharm.2006.07.032. Epub 2006 Sep 1. Neuropharmacology. 2007. PMID: 16949623 - NGF and ProNGF: Regulation of neuronal and neoplastic responses through receptor signaling.
Bradshaw RA, Pundavela J, Biarc J, Chalkley RJ, Burlingame AL, Hondermarck H. Bradshaw RA, et al. Adv Biol Regul. 2015 May;58:16-27. doi: 10.1016/j.jbior.2014.11.003. Epub 2014 Nov 20. Adv Biol Regul. 2015. PMID: 25491371 Free PMC article. Review. - Biogenesis and function of the NGF/TrkA signaling endosome.
Marlin MC, Li G. Marlin MC, et al. Int Rev Cell Mol Biol. 2015;314:239-57. doi: 10.1016/bs.ircmb.2014.10.002. Epub 2014 Nov 18. Int Rev Cell Mol Biol. 2015. PMID: 25619719 Free PMC article. Review.
Cited by
- Identification of a postnatal period of interdependent neurogenesis and apoptosis in peripheral neurons.
Kaminski CL, Banik DD, Schmitd LB, Pierchala BA. Kaminski CL, et al. Biol Open. 2024 Nov 15;13(11):bio060541. doi: 10.1242/bio.060541. Epub 2024 Nov 11. Biol Open. 2024. PMID: 39527572 Free PMC article. - Apoptosis signaling is activated as a transient pulse in neurons.
Spiess KL, Geden MJ, Romero SE, Hollville E, Hammond ES, Patterson RL, Girardi QB, Deshmukh M. Spiess KL, et al. Cell Death Differ. 2024 Oct 26. doi: 10.1038/s41418-024-01403-5. Online ahead of print. Cell Death Differ. 2024. PMID: 39462068 - Enhanced TrkA signaling impairs basal forebrain-dependent behavior.
Calvo-Enrique L, Lisa S, Vicente-García C, Deogracias R, Arévalo JC. Calvo-Enrique L, et al. Front Mol Neurosci. 2023 Sep 22;16:1266983. doi: 10.3389/fnmol.2023.1266983. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37808473 Free PMC article. - Neurotrophic basis to the pathogenesis of depression and phytotherapy.
Wang H, Yang Y, Pei G, Wang Z, Chen N. Wang H, et al. Front Pharmacol. 2023 Apr 6;14:1182666. doi: 10.3389/fphar.2023.1182666. eCollection 2023. Front Pharmacol. 2023. PMID: 37089920 Free PMC article. Review. - High-affinity TrkA and p75 neurotrophin receptor complexes: A twisted affair.
Conroy JN, Coulson EJ. Conroy JN, et al. J Biol Chem. 2022 Mar;298(3):101568. doi: 10.1016/j.jbc.2022.101568. Epub 2022 Jan 17. J Biol Chem. 2022. PMID: 35051416 Free PMC article. Review.
References
- Hamburger V, Levi-Montalcini R. J. Exp. Zool. 1949;111:457. - PubMed
- Levi-Montalcini R. Science. 1987;237:1154. - PubMed
- Crowley C, et al. Cell. 1994;76:1001. - PubMed
- Heerssen HM, Pazyra MF, Segal RA. Nat. Neurosci. 2004;7:596. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F32 NS053187/NS/NINDS NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- EY016281/EY/NEI NIH HHS/United States
- R37 NS034814/NS/NINDS NIH HHS/United States
- NS053187/NS/NINDS NIH HHS/United States
- NS34814/NS/NINDS NIH HHS/United States
- R01 NS034814/NS/NINDS NIH HHS/United States
- R01 EY016281/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials