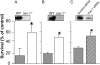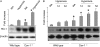Deletion of caveolin-1 protects against oxidative lung injury via up-regulation of heme oxygenase-1 - PubMed (original) (raw)
Deletion of caveolin-1 protects against oxidative lung injury via up-regulation of heme oxygenase-1
Yang Jin et al. Am J Respir Cell Mol Biol. 2008 Aug.
Abstract
Acute lung injury (ALI) is a major cause of morbidity and mortality in critically ill patients. Hyperoxia causes lung injury in animals and humans, and is an established model of ALI. Caveolin-1, a major constituent of caveolae, regulates numerous biological processes, including cell death and proliferation. Here we demonstrate that caveolin-1-null mice (cav-1(-/-)) were resistant to hyperoxia-induced death and lung injury. Cav-1(-/-) mice sustained reduced lung injury after hyperoxia as determined by protein levels in bronchoalveolar lavage fluid and histologic analysis. Furthermore, cav-1(-/-) fibroblasts and endothelial cells and cav-1 knockdown epithelial cells resisted hyperoxia-induced cell death in vitro. Basal and inducible expression of the stress protein heme oxygenase-1 (HO-1) were markedly elevated in lung tissue or fibroblasts from cav-1(-/-) mice. Hyperoxia induced the physical interaction between cav-1 and HO-1 in fibroblasts assessed by co-immunoprecipitation studies, which resulted in attenuation of HO activity. Inhibition of HO activity with tin protoporphyrin-IX abolished the survival benefits of cav-1(-/-) cells and cav-1(-/-) mice exposed to hyperoxia. The cav-1(-/-) mice displayed elevated phospho-p38 mitogen-activated protein kinase (MAPK) and p38beta expression in lung tissue/cells under basal conditions and during hyperoxia. Treatment with SB202190, an inhibitor of p38 MAPK, decreased hyperoxia-inducible HO-1 expression in wild-type and cav-1(-/-) fibroblasts. Taken together, our data demonstrated that cav-1 deletion protects against hyperoxia-induced lung injury, involving in part the modulation of the HO-1-cav-1 interaction, and the enhanced induction of HO-1 through a p38 MAPK-mediated pathway. These studies identify caveolin-1 as a novel component involved in hyperoxia-induced lung injury.
Figures
**Figure 1.
Deletion of caveolin-1 (cav-1) protected against hyperoxia in vitro. Various cell types were exposed to hyperoxia for 72 hours, and cell viability was measured using CellTiter-Glo Luminescent Cell Viability Assay. (A) Fibroblasts were isolated from wild-type and _cav-1_−/− mice. (B) Endothelial cells were isolated from wild-type and _cav-1_−/− mice. (C) Beas-2B cells were transfected with control siRNA or cav-1 siRNA. Shaded bars: wild-type cells or Beas2B cells transfected with control siRNA; open bars: _cav-1_−/− cells; striped bar: cells transfected with cav-1 siRNA. Insets in A and B: Western blot confirming genotype of fibroblasts and endothelial cells. Inset in C: Western blot analysis indicating the efficacy of cav-1 “knock down” in Beas-2B cells. Similar results were found in at least three independent experiments and one representative figure was shown here. Student's _t-_tests were used to determine statistical significance, and the statistical significance was defined as P < 0.05. Furthermore, cell viability was measured using trypan blue exclusion assays. (D) The same fibroblasts from experiment in A were used. (E) Beas-2B cells were transfected with control siRNA or cav-1 siRNA as described above in C. Shaded bars: wild-type cells or Beas2B cells transfected with control siRNA; open bar: _cav-1_−/− cells; striped bar: cells transfected with cav-1 siRNA. All figures represented at least three independent experiments. (F) Gain-of-function experiment. Wild-type fibroblasts were infected with _ad-cav-1_and ad-lacZ as described in M
aterials and
M
ethods
. After 24 hours of hyperoxia, cell viability was measured. Shaded bar: cells infected with ad-lacZ; open bar: cells infected with ad-cav-1. Inset: Western blot analysis indicating the overexpressing cav-1 in wild-type fibroblasts infected with ad-cav-1. Figures represented at least three independent experiments. In each experiment, each point had triplicate samples. *Statistically significant (P < 0.05, Student's t test).
**Figure 1.
Deletion of caveolin-1 (cav-1) protected against hyperoxia in vitro. Various cell types were exposed to hyperoxia for 72 hours, and cell viability was measured using CellTiter-Glo Luminescent Cell Viability Assay. (A) Fibroblasts were isolated from wild-type and _cav-1_−/− mice. (B) Endothelial cells were isolated from wild-type and _cav-1_−/− mice. (C) Beas-2B cells were transfected with control siRNA or cav-1 siRNA. Shaded bars: wild-type cells or Beas2B cells transfected with control siRNA; open bars: _cav-1_−/− cells; striped bar: cells transfected with cav-1 siRNA. Insets in A and B: Western blot confirming genotype of fibroblasts and endothelial cells. Inset in C: Western blot analysis indicating the efficacy of cav-1 “knock down” in Beas-2B cells. Similar results were found in at least three independent experiments and one representative figure was shown here. Student's _t-_tests were used to determine statistical significance, and the statistical significance was defined as P < 0.05. Furthermore, cell viability was measured using trypan blue exclusion assays. (D) The same fibroblasts from experiment in A were used. (E) Beas-2B cells were transfected with control siRNA or cav-1 siRNA as described above in C. Shaded bars: wild-type cells or Beas2B cells transfected with control siRNA; open bar: _cav-1_−/− cells; striped bar: cells transfected with cav-1 siRNA. All figures represented at least three independent experiments. (F) Gain-of-function experiment. Wild-type fibroblasts were infected with _ad-cav-1_and ad-lacZ as described in M
aterials and
M
ethods
. After 24 hours of hyperoxia, cell viability was measured. Shaded bar: cells infected with ad-lacZ; open bar: cells infected with ad-cav-1. Inset: Western blot analysis indicating the overexpressing cav-1 in wild-type fibroblasts infected with ad-cav-1. Figures represented at least three independent experiments. In each experiment, each point had triplicate samples. *Statistically significant (P < 0.05, Student's t test).
**Figure 1.
Deletion of caveolin-1 (cav-1) protected against hyperoxia in vitro. Various cell types were exposed to hyperoxia for 72 hours, and cell viability was measured using CellTiter-Glo Luminescent Cell Viability Assay. (A) Fibroblasts were isolated from wild-type and _cav-1_−/− mice. (B) Endothelial cells were isolated from wild-type and _cav-1_−/− mice. (C) Beas-2B cells were transfected with control siRNA or cav-1 siRNA. Shaded bars: wild-type cells or Beas2B cells transfected with control siRNA; open bars: _cav-1_−/− cells; striped bar: cells transfected with cav-1 siRNA. Insets in A and B: Western blot confirming genotype of fibroblasts and endothelial cells. Inset in C: Western blot analysis indicating the efficacy of cav-1 “knock down” in Beas-2B cells. Similar results were found in at least three independent experiments and one representative figure was shown here. Student's _t-_tests were used to determine statistical significance, and the statistical significance was defined as P < 0.05. Furthermore, cell viability was measured using trypan blue exclusion assays. (D) The same fibroblasts from experiment in A were used. (E) Beas-2B cells were transfected with control siRNA or cav-1 siRNA as described above in C. Shaded bars: wild-type cells or Beas2B cells transfected with control siRNA; open bar: _cav-1_−/− cells; striped bar: cells transfected with cav-1 siRNA. All figures represented at least three independent experiments. (F) Gain-of-function experiment. Wild-type fibroblasts were infected with _ad-cav-1_and ad-lacZ as described in M
aterials and
M
ethods
. After 24 hours of hyperoxia, cell viability was measured. Shaded bar: cells infected with ad-lacZ; open bar: cells infected with ad-cav-1. Inset: Western blot analysis indicating the overexpressing cav-1 in wild-type fibroblasts infected with ad-cav-1. Figures represented at least three independent experiments. In each experiment, each point had triplicate samples. *Statistically significant (P < 0.05, Student's t test).
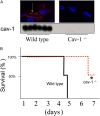
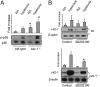
**Figure 2.
Deletion of cav-1 protected against hyperoxia in vivo. (A) Immunofluorescence and Western blot analysis were performed to evaluate the expression of cav-1 in lung tissue from wild-type and _cav-1_−/− mice. Red stain indicates cav-1. (B) Survival of mice after continuous hyperoxia treatment. _X_-axis: days after hyperoxia; _y_-axis: percentage of survived mice. Solid line: wild-type mice; dotted line: cav-1_−/− mice (n = 22; 11 wild-type mice and 11 cav-1_−/− mice exposed to hyperoxia). The ages of cav-1−/− mice and wild-type mice were between 8 and 12 weeks. In each experiment, we matched the age of wild-type mice and cav-1−/− mice. The difference of ages between the wild-type mice and cav-1−/− mice was 2 days. All wild-type mice died before 120 hours (5 d), no exclusions. *Statistically significant (*P < 0.05; Chi-square test).
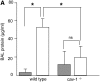
**Figure 3.
Deletion of cav-1 reduced lung injury after hyperoxia. (A) Mice were exposed to hyperoxia for 96 hours. Bronchoalveolar lavage fluid was obtained from wild-type and hyperoxia-treated mice, and protein concentration was determined. Shaded bars: mice treated with room air; open bars: mice treated with hyperoxia. *P < 0.05. Four mice in each group were used. Student's t tests were used. Statistical significance was defined as P < 0.05. (B) Lung cellular morphology was examined by transition electronic microscopy (TEM). Upper panels: mice treated with room air. Lower panels: mice exposed to hyperoxia. Left panels: wild-type mice. Right panels: _cav-1_−/− mice. Black arrows: nuclei. Representative figures are shown.
**Figure 3.
Deletion of cav-1 reduced lung injury after hyperoxia. (A) Mice were exposed to hyperoxia for 96 hours. Bronchoalveolar lavage fluid was obtained from wild-type and hyperoxia-treated mice, and protein concentration was determined. Shaded bars: mice treated with room air; open bars: mice treated with hyperoxia. *P < 0.05. Four mice in each group were used. Student's t tests were used. Statistical significance was defined as P < 0.05. (B) Lung cellular morphology was examined by transition electronic microscopy (TEM). Upper panels: mice treated with room air. Lower panels: mice exposed to hyperoxia. Left panels: wild-type mice. Right panels: _cav-1_−/− mice. Black arrows: nuclei. Representative figures are shown.
**Figure 4.
Expression and regulation of heme oxygenase-1 (HO-1) in wild-type and _cav-1_−/− mice. (A) Wild-type and cav-1−/− mice were subjected to hyperoxia in vivo. After 96 hours, lung tissue was excised, homogenized, and analyzed for HO-1 expression by Western blot analysis. Figures represented three independent experiments. *P < 0.05 (statistical analysis was performed using t tests). (B) Time course of hyperoxia-induced HO-1 expression in wild-type and _cav-1_−/− mice. Wild-type and cav-1−/− mice were subjected to hyperoxia in vivo for different days as indicated. Lung tissue was excised, homogenized, and analyzed for HO-1 expression by Western blot analysis. There were no wild-type mice that survived on Day 5 from which to collect lung tissue for Western blot analysis. Figures represented three independent experiments. *P < 0.05 (statistics performed using t tests on time [day]-matched samples). (C) Wild-type and cav-1−/− fibroblasts were exposed to hyperoxia (95% O2, 5% CO2) in vitro for 0 to 72 hours. HO-1 expression was determined by Western blot analysis. Figures represented three independent experiments. *P < 0.05 (statistics performed using t test on time-matched samples). (D) Wild-type fibroblasts were treated with hyperoxia (72 h). The interaction of HO-1 and cav-1 was detected by immunoprecipitation with HO-1 polyclonal antibodies followed by blotting with cav-1 monoclonal antibodies. Figure represented three independent experiments. (E) HO-1 activity in wild-type (open bars) and cav-1−/− (shaded bars) fibroblasts with and without hyperoxia. Wild-type and cav-1−/− fibroblasts were exposed to hyperoxia (95% O2, 5% CO2) in vitro. After 72 hours, HO-1 activity was measured as described in M
aterials and
M
ethods
. Open bars: wild-type cells; shaded bars: cav-1−/− cells. Figure represents three independent experiments. *P < 0.05 (statistics performed using t tests).
**Figure 4.
Expression and regulation of heme oxygenase-1 (HO-1) in wild-type and _cav-1_−/− mice. (A) Wild-type and cav-1−/− mice were subjected to hyperoxia in vivo. After 96 hours, lung tissue was excised, homogenized, and analyzed for HO-1 expression by Western blot analysis. Figures represented three independent experiments. *P < 0.05 (statistical analysis was performed using t tests). (B) Time course of hyperoxia-induced HO-1 expression in wild-type and _cav-1_−/− mice. Wild-type and cav-1−/− mice were subjected to hyperoxia in vivo for different days as indicated. Lung tissue was excised, homogenized, and analyzed for HO-1 expression by Western blot analysis. There were no wild-type mice that survived on Day 5 from which to collect lung tissue for Western blot analysis. Figures represented three independent experiments. *P < 0.05 (statistics performed using t tests on time [day]-matched samples). (C) Wild-type and cav-1−/− fibroblasts were exposed to hyperoxia (95% O2, 5% CO2) in vitro for 0 to 72 hours. HO-1 expression was determined by Western blot analysis. Figures represented three independent experiments. *P < 0.05 (statistics performed using t test on time-matched samples). (D) Wild-type fibroblasts were treated with hyperoxia (72 h). The interaction of HO-1 and cav-1 was detected by immunoprecipitation with HO-1 polyclonal antibodies followed by blotting with cav-1 monoclonal antibodies. Figure represented three independent experiments. (E) HO-1 activity in wild-type (open bars) and cav-1−/− (shaded bars) fibroblasts with and without hyperoxia. Wild-type and cav-1−/− fibroblasts were exposed to hyperoxia (95% O2, 5% CO2) in vitro. After 72 hours, HO-1 activity was measured as described in M
aterials and
M
ethods
. Open bars: wild-type cells; shaded bars: cav-1−/− cells. Figure represents three independent experiments. *P < 0.05 (statistics performed using t tests).
**Figure 4.
Expression and regulation of heme oxygenase-1 (HO-1) in wild-type and _cav-1_−/− mice. (A) Wild-type and cav-1−/− mice were subjected to hyperoxia in vivo. After 96 hours, lung tissue was excised, homogenized, and analyzed for HO-1 expression by Western blot analysis. Figures represented three independent experiments. *P < 0.05 (statistical analysis was performed using t tests). (B) Time course of hyperoxia-induced HO-1 expression in wild-type and _cav-1_−/− mice. Wild-type and cav-1−/− mice were subjected to hyperoxia in vivo for different days as indicated. Lung tissue was excised, homogenized, and analyzed for HO-1 expression by Western blot analysis. There were no wild-type mice that survived on Day 5 from which to collect lung tissue for Western blot analysis. Figures represented three independent experiments. *P < 0.05 (statistics performed using t tests on time [day]-matched samples). (C) Wild-type and cav-1−/− fibroblasts were exposed to hyperoxia (95% O2, 5% CO2) in vitro for 0 to 72 hours. HO-1 expression was determined by Western blot analysis. Figures represented three independent experiments. *P < 0.05 (statistics performed using t test on time-matched samples). (D) Wild-type fibroblasts were treated with hyperoxia (72 h). The interaction of HO-1 and cav-1 was detected by immunoprecipitation with HO-1 polyclonal antibodies followed by blotting with cav-1 monoclonal antibodies. Figure represented three independent experiments. (E) HO-1 activity in wild-type (open bars) and cav-1−/− (shaded bars) fibroblasts with and without hyperoxia. Wild-type and cav-1−/− fibroblasts were exposed to hyperoxia (95% O2, 5% CO2) in vitro. After 72 hours, HO-1 activity was measured as described in M
aterials and
M
ethods
. Open bars: wild-type cells; shaded bars: cav-1−/− cells. Figure represents three independent experiments. *P < 0.05 (statistics performed using t tests).
**Figure 5.
HO activity is required for hyperoxia resistance in cav-1–deficient cells and mice. (A) Wild-type and cav-1−/− cells were subjected to hyperoxia (95% O2, 5% CO2) after pretreatment with SnPP (20 μM; open bars) or vehicle (DMSO; shaded bars). After 72 hours, cell viability was evaluated as previously described. The percentage of surviving cells after hyperoxia was determined. Shaded bars: cells pre-treated with DMSO; open bars: cells pre-treated with SnPP. Results reflect the mean and standard deviation of three determinations (*P < 0.05). Figure represents three independent experiments. Statistical analysis was performed using t tests. (B) SnPP was administered to wild-type or _cav-1_−/− mice by injection (20 μmol/kg/d, intraperitoneally). PBS with the same volume was used as control. The animals were exposed to room air or hyperoxia (95% O2, 5% N2) and time-dependent survival was determined (n = 7 in each group). *P < 0.05 (statistics performed using chi-square tests [categorical data]).
**Figure 6.
Hyperoxia-inducible HO-1 expression requires p38 mitogen-activated protein kinase (MAPK) in wild-type and _cav-1_−/− mice. (A) Expression of phosphor-p38 (p-p38) in wild-type or _cav-1_−/− mice. Wild-type and _cav-1_−/− mice were exposed to room air (RA) or hyperoxia (95% O2, 5% N2) for 96 hours. Total or phospho-p38 MAPK levels were determined by Western blot analysis of lung tissue homogenates. Figure represents three independent experiments (statistics performed using t tests; *P < 0.05). (B) Wild-type or _cav-1_−/− fibroblasts were pretreated with DMSO or SB202190 (10 μM) and then exposed to hyperoxia (95% O2, 5% CO2) or room air (RA) for 72 hours. HO-1 expression was determined by Western blot analysis. Figure represents three independent experiments (statistics performed using t tests; *P < 0.05). (C) Expression of p38 isoforms in wild-type or _cav-1_−/− fibroblasts. Wild-type and _cav-1_−/− fibroblasts were exposed to room air (RA) or hyperoxia (95% O2, 5% N2) for 24 hours. Western blot analysis was performed to determine the expression of p-38 isoforms. Figures represent three independent experiments (statistics performed using t tests; *P < 0.05).
**Figure 6.
Hyperoxia-inducible HO-1 expression requires p38 mitogen-activated protein kinase (MAPK) in wild-type and _cav-1_−/− mice. (A) Expression of phosphor-p38 (p-p38) in wild-type or _cav-1_−/− mice. Wild-type and _cav-1_−/− mice were exposed to room air (RA) or hyperoxia (95% O2, 5% N2) for 96 hours. Total or phospho-p38 MAPK levels were determined by Western blot analysis of lung tissue homogenates. Figure represents three independent experiments (statistics performed using t tests; *P < 0.05). (B) Wild-type or _cav-1_−/− fibroblasts were pretreated with DMSO or SB202190 (10 μM) and then exposed to hyperoxia (95% O2, 5% CO2) or room air (RA) for 72 hours. HO-1 expression was determined by Western blot analysis. Figure represents three independent experiments (statistics performed using t tests; *P < 0.05). (C) Expression of p38 isoforms in wild-type or _cav-1_−/− fibroblasts. Wild-type and _cav-1_−/− fibroblasts were exposed to room air (RA) or hyperoxia (95% O2, 5% N2) for 24 hours. Western blot analysis was performed to determine the expression of p-38 isoforms. Figures represent three independent experiments (statistics performed using t tests; *P < 0.05).
Similar articles
- Caveolin-1: a critical regulator of lung injury.
Jin Y, Lee SJ, Minshall RD, Choi AM. Jin Y, et al. Am J Physiol Lung Cell Mol Physiol. 2011 Feb;300(2):L151-60. doi: 10.1152/ajplung.00170.2010. Epub 2010 Nov 19. Am J Physiol Lung Cell Mol Physiol. 2011. PMID: 21097526 Free PMC article. Review. - Deletion of caveolin-1 protects hyperoxia-induced apoptosis via survivin-mediated pathways.
Zhang M, Lin L, Lee SJ, Mo L, Cao J, Ifedigbo E, Jin Y. Zhang M, et al. Am J Physiol Lung Cell Mol Physiol. 2009 Nov;297(5):L945-53. doi: 10.1152/ajplung.00081.2009. Epub 2009 Sep 18. Am J Physiol Lung Cell Mol Physiol. 2009. PMID: 19767411 Free PMC article. - [Research on the effect of protection against ventilator-induced lung injury via regulation of caveolin-1/heme oxygenase-1 signaling].
Zhong R, Xiao J, Yu Z, Zhou J, Dai C. Zhong R, et al. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2015 Jul;27(7):568-73. doi: 10.3760/cma.j.issn.2095-4352.2015.07.006. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2015. PMID: 26138418 Chinese. - Caveolin-1 scaffolding domain peptides enhance anti-inflammatory effect of heme oxygenase-1 through interrupting its interact with caveolin-1.
Weng P, Zhang XT, Sheng Q, Tian WF, Chen JL, Yuan JJ, Zhang JR, Pang QF. Weng P, et al. Oncotarget. 2017 Jun 20;8(25):40104-40114. doi: 10.18632/oncotarget.16676. Oncotarget. 2017. PMID: 28402952 Free PMC article. - Heme oxygenase-1/carbon monoxide: novel therapeutic strategies in critical care medicine.
Ryter SW, Choi AM. Ryter SW, et al. Curr Drug Targets. 2010 Dec;11(12):1485-94. doi: 10.2174/1389450111009011485. Curr Drug Targets. 2010. PMID: 20704552 Review.
Cited by
- Caveolin-1 inhibits expression of antioxidant enzymes through direct interaction with nuclear erythroid 2 p45-related factor-2 (Nrf2).
Li W, Liu H, Zhou JS, Cao JF, Zhou XB, Choi AM, Chen ZH, Shen HH. Li W, et al. J Biol Chem. 2012 Jun 15;287(25):20922-30. doi: 10.1074/jbc.M112.352336. Epub 2012 Apr 30. J Biol Chem. 2012. PMID: 22547061 Free PMC article. - Autophagy protein microtubule-associated protein 1 light chain-3B (LC3B) activates extrinsic apoptosis during cigarette smoke-induced emphysema.
Chen ZH, Lam HC, Jin Y, Kim HP, Cao J, Lee SJ, Ifedigbo E, Parameswaran H, Ryter SW, Choi AM. Chen ZH, et al. Proc Natl Acad Sci U S A. 2010 Nov 2;107(44):18880-5. doi: 10.1073/pnas.1005574107. Epub 2010 Oct 18. Proc Natl Acad Sci U S A. 2010. PMID: 20956295 Free PMC article. - HMSCs exosome-derived miR-199a-5p attenuates sulfur mustard-associated oxidative stress via the CAV1/NRF2 signalling pathway.
Gong C, Gu Z, Zhang X, Xu Q, Mao G, Pei Z, Meng W, Cen J, Liu J, He X, Sun M, Xiao K. Gong C, et al. J Cell Mol Med. 2023 Aug;27(15):2165-2182. doi: 10.1111/jcmm.17803. Epub 2023 Jun 29. J Cell Mol Med. 2023. PMID: 37386746 Free PMC article. - AICAR decreases acute lung injury by phosphorylating AMPK and upregulating heme oxygenase-1.
Ahmad I, Molyvdas A, Jian MY, Zhou T, Traylor AM, Cui H, Liu G, Song W, Agarwal A, Jilling T, Aggarwal S, Matalon S. Ahmad I, et al. Eur Respir J. 2021 Dec 23;58(6):2003694. doi: 10.1183/13993003.03694-2020. Print 2021 Dec. Eur Respir J. 2021. PMID: 34049949 Free PMC article. - Caveolin-1: a critical regulator of lung injury.
Jin Y, Lee SJ, Minshall RD, Choi AM. Jin Y, et al. Am J Physiol Lung Cell Mol Physiol. 2011 Feb;300(2):L151-60. doi: 10.1152/ajplung.00170.2010. Epub 2010 Nov 19. Am J Physiol Lung Cell Mol Physiol. 2011. PMID: 21097526 Free PMC article. Review.
References
- Wheeler AP, Bernard GR. Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet 2007;369:1553–1564. - PubMed
- Steinberg KP, Hudson LD. Acute lung injury and acute respiratory distress syndrome: the clinical syndrome. Clin Chest Med 2000;21:401–417. - PubMed
- Horowitz S, Davis JM. Lung injury when development is interrupted by premature birth. In: Lung growth and development. McDonald JA, editor New York: Dekker, 1997. pp. 577–610.
- Crapo JD. Morphologic changes in pulmonary oxygen toxicity. Annu Rev Physiol 1986;48:721–731. - PubMed
- Mantell LL, Lee PJ. Signal transduction pathways in hyperoxia-induced lung cell death. Mol Genet Metab 2000;71:359–370. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K08 HL085601/HL/NHLBI NIH HHS/United States
- R01-HL079904/HL/NHLBI NIH HHS/United States
- R01-HL55330/HL/NHLBI NIH HHS/United States
- K08 HL 085601-01/HL/NHLBI NIH HHS/United States
- P01 HL70807/HL/NHLBI NIH HHS/United States
- R01-HL60234/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases