Combined targeting of epidermal growth factor receptor, signal transducer and activator of transcription-3, and Bcl-X(L) enhances antitumor effects in squamous cell carcinoma of the head and neck - PubMed (original) (raw)
Comparative Study
. 2008 Jun;73(6):1632-42.
doi: 10.1124/mol.107.044636. Epub 2008 Mar 6.
Affiliations
- PMID: 18326051
- PMCID: PMC3437602
- DOI: 10.1124/mol.107.044636
Comparative Study
Combined targeting of epidermal growth factor receptor, signal transducer and activator of transcription-3, and Bcl-X(L) enhances antitumor effects in squamous cell carcinoma of the head and neck
Amanda L Boehm et al. Mol Pharmacol. 2008 Jun.
Abstract
Squamous cell carcinoma of the head and neck (SCCHN) is a leading cause of cancer deaths worldwide. Epidermal growth factor receptor (EGFR), an upstream mediator of signal transducer and activator of transcription (STAT)-3 is overexpressed in a variety of cancers, including SCCHN. Therapies such as monoclonal antibodies and tyrosine kinase inhibitors targeting EGFR have demonstrated limited antitumor efficacy, which may be explained, in part, by persistent STAT3 activation despite EGFR inhibition. STAT3 activation induces expression of target genes in SCCHN, including Bcl-X(L), a mediator of antiapoptotic activity. Bcl-X(L) is commonly overexpressed in SCCHN where it correlates with chemoresistance, making it a potential therapeutic target. Targeting the EGFR-STAT3-Bcl-X(L) pathway at several levels, including the upstream receptor, the intracellular transcription factor, and the downstream target gene, has not been investigated previously. Using erlotinib, an EGFR-specific reversible tyrosine kinase inhibitor in combination with a STAT3 transcription factor decoy, we found enhanced antitumor effects in vitro and in vivo. The combination of the STAT3 decoy and gossypol, a small molecule targeting Bcl-X(L), also yielded enhanced inhibition of cell proliferation. The triple combination of erlotinib, STAT3 decoy, and gossypol further enhanced cell growth inhibition and apoptosis in vitro, and it down-regulated signaling molecules further downstream of the EGFR-STAT3 signaling pathway, such as cyclin D1. These results suggest that combined targeting of several components of an oncogenic signaling pathway may be an effective therapeutic strategy for SCCHN.
Figures
Fig. 1
Combining the STAT3 decoy with erlotinib enhances inhibition of cell viability in vitro. A, UM-22B cells were transfected with 12.6 nM STAT3 decoy or mutant control decoy for 4 h. Transfection media were removed, and DMEM containing 10% FBS and 5 µM erlotinib was added. Cells were then counted after 72 h using trypan blue dye exclusion. When the STAT3 decoy was combined with erlotinib, cell viability was significantly reduced compared with STAT3 decoy alone (p = 0.004), erlotinib alone (p = 0.024), or erlotinib plus mutant control decoy (p = 0.028). B, similar results were seen for PCI-15B cells (p = 0.004, p = 0.024, and p = 0.016) when treated with 0.1 µM erlotinib and 38.3 nM STAT3 decoy. Cumulative results are shown from five separate experiments.
Fig. 2
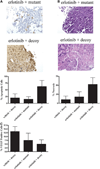
Combining the STAT3 decoy with erlotinib enhances cell growth inhibition in vivo. We inoculated 1483 cells (2 × 106) subcutaneously in the right and left flanks of 11 athymic nude mice. After 14 days, the tumors were palpable, and mice were randomized into two treatment groups. Tumors on the left flank were injected with the mutant control decoy, and tumors on the right flank were treated with the STAT3 decoy (35 µg) daily for 31 days. In addition, five mice received 90 mg/kg erlotinib, and six mice received vehicle control by oral gavage. Tumors were harvested at the end of treatment (day 31), and they were stained for apoptosis using a TUNEL assay (A) or for necrosis (B), by hematoxylin and eosin staining, and VEGF expression (C). The average percentage of apoptotic, necrotic, and VEGF-positive cells are represented. The percentage of apoptotic cells by TUNEL excluded the areas of necrosis as part of the denominator.
Fig. 3
Combination of the STAT3 decoy with gossypol enhances inhibition of cell viability of head and neck cancer cells. A, UM-22B cells were treated with 12.6 nM STAT3 decoy or mutant control decoy for 4 h followed by treatment with 2.67 µM gossypol for 72 h. Cell counts were then performed using trypan blue dye exclusion. Combining the STAT3 decoy with gossypol augmented inhibition of cell viability compared with STAT3 decoy alone, gossypol alone, gossypol alone, or mutant control plus gossypol (p = 0.075, p = 0.2, and p = 0.155, respectively). B, similar results were seen when PCI-15B cells were treated with 38.3 nM STAT3 decoy and 2.97 µM gossypol (p = 0.0278, p = 0.5, and p = 0.0278). Cumulative results are shown from five separate experiments.
Fig. 4
Combining erlotinib, STAT3 decoy, and gossypol further enhances inhibition of cell viability in SCCHN cells. A, UM-22B cells were plated in 96-well plates, and then they were treated with 5 µM erlotinib, 12.6 nM STAT3 decoy, and 2.67 µM gossypol. Cell counts were performed by trypan blue dye exclusion at 72 h. The combination of erlotinib, STAT3 decoy, and gossypol enhanced inhibition of cell viability compared with STAT3 decoy alone (p = 0.004), erlotinib plus gossypol (p = 0.05), or the combination of erlotinib, mutant control, and gossypol (p = 0.0476). B, similar results were seen with PCI-15B treated with 0.16 µM erlotinib, 38.3 nM STAT3 decoy, and 2.97 µM gossypol (p = 0.004, p = 0.02, and p = 0.008, respectively). Cumulative results are shown from five separate experiments.
Fig. 5
Combining erlotinib, STAT3 decoy, and gossypol induces apoptosis in vitro. UM-22B (A) and PCI-15B (B) cells were treated with STAT3 decoy/control decoy (12.6 and 38.3 nM, respectively), erlotinib (5 and 0.16 µM, respectively), and gossypol (2.67 and 2.97 µM, respectively) alone or in combination for 24 h, followed by annexin V assay. Percentage of apoptotic cells is expressed as mean ± S.E.M.
Fig. 6
Combining erlotinib, STAT3 decoy, and gossypol inhibits the p44/42 MAPK and mammalian target of rapamycin pathways, as well as cyclin D1 and p-Akt protein expression in vitro. PCI-15B cells were treated with STAT3 decoy/control decoy (38.3 nM), erlotinib (0.16 µM), and/or gossypol (2.97 µM) alone or in combination for 24 h. A, lysates were collected, and 40 µg of protein/lane was immunoblotted for cyclin D1, phospho-p44/42 MAPK, p44/42 MAPK, phospho-p70S6 kinase, p70S6 kinase, p-Akt, Akt, and β-tubulin (loading control). Results shown are representative of three independent experiments. B to E are presentations of densitometry data of PCI-15B cells treated with decoy/control decoy, erlotinib, and gossypol (alone or in combination), and the data are expressed as a ratio with respect to β-tubulin or total protein. Protein levels are expressed as mean ± S.E.M. The results represent densitometry performed on Western blots from three independent experiments.
Similar articles
- Honokiol inhibits epidermal growth factor receptor signaling and enhances the antitumor effects of epidermal growth factor receptor inhibitors.
Leeman-Neill RJ, Cai Q, Joyce SC, Thomas SM, Bhola NE, Neill DB, Arbiser JL, Grandis JR. Leeman-Neill RJ, et al. Clin Cancer Res. 2010 May 1;16(9):2571-9. doi: 10.1158/1078-0432.CCR-10-0333. Epub 2010 Apr 13. Clin Cancer Res. 2010. PMID: 20388852 Free PMC article. - Targeting Stat3 abrogates EGFR inhibitor resistance in cancer.
Sen M, Joyce S, Panahandeh M, Li C, Thomas SM, Maxwell J, Wang L, Gooding WE, Johnson DE, Grandis JR. Sen M, et al. Clin Cancer Res. 2012 Sep 15;18(18):4986-96. doi: 10.1158/1078-0432.CCR-12-0792. Epub 2012 Jul 23. Clin Cancer Res. 2012. PMID: 22825581 Free PMC article. - In Vitro and In Vivo Synergistic Antitumor Activity of the Combination of BKM120 and Erlotinib in Head and Neck Cancer: Mechanism of Apoptosis and Resistance.
Anisuzzaman AS, Haque A, Wang D, Rahman MA, Zhang C, Chen Z, Chen ZG, Shin DM, Amin AR. Anisuzzaman AS, et al. Mol Cancer Ther. 2017 Apr;16(4):729-738. doi: 10.1158/1535-7163.MCT-16-0683. Epub 2017 Jan 23. Mol Cancer Ther. 2017. PMID: 28119490 - New approaches to EGFR inhibition for locally advanced or metastatic squamous cell carcinoma of the head and neck (SCCHN).
Agulnik M. Agulnik M. Med Oncol. 2012 Dec;29(4):2481-91. doi: 10.1007/s12032-012-0159-2. Epub 2012 Jan 18. Med Oncol. 2012. PMID: 22252310 Free PMC article. Review. - The STAT3 pathway as a therapeutic target in head and neck cancer: Barriers and innovations.
Geiger JL, Grandis JR, Bauman JE. Geiger JL, et al. Oral Oncol. 2016 May;56:84-92. doi: 10.1016/j.oraloncology.2015.11.022. Epub 2015 Dec 28. Oral Oncol. 2016. PMID: 26733183 Free PMC article. Review.
Cited by
- STAT3 contributes to NK cell recognition by modulating expression of NKG2D ligands in adriamycin-resistant K562/AO2 cells.
Cai X, Lu X, Jia Z, Zhang X, Han W, Rong X, Ma L, Zhou M, Chen B. Cai X, et al. Int J Hematol. 2015 Nov;102(5):536-43. doi: 10.1007/s12185-015-1860-7. Epub 2015 Sep 19. Int J Hematol. 2015. PMID: 26387089 - The anti-diabetic drug exenatide, a glucagon-like peptide-1 receptor agonist, counteracts hepatocarcinogenesis through cAMP-PKA-EGFR-STAT3 axis.
Zhou M, Mok MT, Sun H, Chan AW, Huang Y, Cheng AS, Xu G. Zhou M, et al. Oncogene. 2017 Jul 20;36(29):4135-4149. doi: 10.1038/onc.2017.38. Epub 2017 Mar 20. Oncogene. 2017. PMID: 28319060 - Targeting the JAK/STAT Signaling Pathway Using Phytocompounds for Cancer Prevention and Therapy.
Bose S, Banerjee S, Mondal A, Chakraborty U, Pumarol J, Croley CR, Bishayee A. Bose S, et al. Cells. 2020 Jun 11;9(6):1451. doi: 10.3390/cells9061451. Cells. 2020. PMID: 32545187 Free PMC article. Review. - Induction of BCL2-Interacting Killer, BIK, is Mediated for Anti-Cancer Activity of Curcumin in Human Head and Neck Squamous Cell Carcinoma Cells.
Xi Y, Gao H, Callaghan MU, Fribley AM, Garshott DM, Xu ZX, Zeng Q, Li YL. Xi Y, et al. J Cancer. 2015 Feb 12;6(4):327-32. doi: 10.7150/jca.11185. eCollection 2015. J Cancer. 2015. PMID: 25767602 Free PMC article. - Overexpression of Bcl-2 induces STAT-3 activation via an increase in mitochondrial superoxide.
Kang J, Chong SJ, Ooi VZ, Vali S, Kumar A, Kapoor S, Abbasi T, Hirpara JL, Loh T, Goh BC, Pervaiz S. Kang J, et al. Oncotarget. 2015 Oct 27;6(33):34191-205. doi: 10.18632/oncotarget.5763. Oncotarget. 2015. PMID: 26430964 Free PMC article.
References
- Ahonen TJ, Xie J, LeBaron MJ, Zhu J, Nurmi M, Alanen K, Rui H, Nevalainen MT. Inhibition of transcription factor Stat5 induces cell death of human prostate cancer cells. J Biol Chem. 2003;278:27287–27292. - PubMed
- Ala-aho R, Ahonen M, George SJ, Heikkila J, Grenman R, Kallajoki M, Kahari VM. Targeted inhibition of human collagenase-3 (MMP-13) expression inhibits squamous cell carcinoma growth in vivo. Oncogene. 2004;23:5111–5123. - PubMed
- Ang KK, Berkey BA, Tu X, Zhang HZ, Katz R, Hammond EH, Fu KK, Milas L. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002;62:7350–7356. - PubMed
- Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2R01-CA77308/CA/NCI NIH HHS/United States
- P50-CA097190-01A1/CA/NCI NIH HHS/United States
- R01 CA077308/CA/NCI NIH HHS/United States
- P50 CA097190/CA/NCI NIH HHS/United States
- R01 CA101840/CA/NCI NIH HHS/United States
- P30 EY008098/EY/NEI NIH HHS/United States
- EY08098/EY/NEI NIH HHS/United States
- R01-CA101840/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous