Three-dimensional resolution doubling in wide-field fluorescence microscopy by structured illumination - PubMed (original) (raw)
Three-dimensional resolution doubling in wide-field fluorescence microscopy by structured illumination
Mats G L Gustafsson et al. Biophys J. 2008 Jun.
Abstract
Structured illumination microscopy is a method that can increase the spatial resolution of wide-field fluorescence microscopy beyond its classical limit by using spatially structured illumination light. Here we describe how this method can be applied in three dimensions to double the axial as well as the lateral resolution, with true optical sectioning. A grating is used to generate three mutually coherent light beams, which interfere in the specimen to form an illumination pattern that varies both laterally and axially. The spatially structured excitation intensity causes normally unreachable high-resolution information to become encoded into the observed images through spatial frequency mixing. This new information is computationally extracted and used to generate a three-dimensional reconstruction with twice as high resolution, in all three dimensions, as is possible in a conventional wide-field microscope. The method has been demonstrated on both test objects and biological specimens, and has produced the first light microscopy images of the synaptonemal complex in which the lateral elements are clearly resolved.
Figures
FIGURE 1
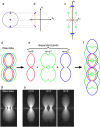
Enlargement of the observable region of reciprocal space through structured illumination. (a–e) Observable regions for (a and b) the conventional microscope, and for structured illumination microscopy using two illumination beams (c), and three illumination beams in one (d) or three (e) sequential orientations. (f) The three amplitude wave vectors corresponding to the three illumination beam directions. All three wave vectors have the same magnitude 1/λ. (g–h) The resulting spatial frequency components of the illumination intensity for the two-beam (g) and three-beam (h) case. The dotted outline in panel h indicates the set of spatial frequencies that are possible to generate by illumination through the objective lens; compare with the observable region in panel a. An intensity component occurs at each pairwise difference frequency between two of the amplitude wave vectors. (i, j): x z (i) and x y (j) sections through the OTF supports in panel b (shown in white), panel c (light shaded), panel d (dark shaded), and panel e (solid). The darker regions fully contain the lighter ones.
FIGURE 2
The optical transfer functions _O_m and the effect of incoherence. (a) The location of the three illumination beams (purple dots) in the back focal plane of the objective lens. Each point within each beam is mutually coherent only with the corresponding point of each of the other beams (which originates from the same point of the light source); the yellow points illustrate one such coherent point triplet. (b) Spatial frequency components of the light amplitude. For an objective lens that satisfies the Sine Condition, the pupil plane maps directly onto the lateral spatial frequency (dotted lines from a to b). The difference vectors between mutually coherent amplitude components (amber arrows in b) will therefore have the same lateral extent, but will differ axially, for different source points. (c) Resulting spatial frequency components of the illumination intensity; the m = 0, ±1, and ±2 components are shown in red, green, and blue, respectively. The non-zero-m components, which get a contribution from each coherent point pair (amber arrows, corresponding to those in b), are axially broadened into line segments, but remain delta-function-like laterally. The raw data (d and g) is a sum of five information bands, corresponding to the five lateral illumination-intensity frequencies. Once five images are observed with different pattern phases, the bands can be separated (e and h; only the m = 0 (red), 1 (green), and 2 (blue) bands are shown), and computationally moved back to their correct lateral position (f). Each band has been broadened by convolution with the corresponding frequency component of the illumination intensity (corresponding colors); the order 1 component, for example, consists of two parts (e, green). The observed raw data (g) and separated bands (h) for a point-source object are in excellent agreement with the theoretical expectations.
FIGURE 3
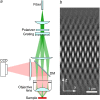
(a) Simplified diagram of the structured illumination apparatus. Scrambled laser light from a multimode fiber is collimated onto a linear phase grating. Diffraction orders –1, 0 and +1 are refocused into the back focal plane of an objective lens. The beams, recollimated by the objective lens, intersect at the focal plane in the sample, where they interfere and generate an intensity pattern with both lateral and axial structure (b). The finite axial extent of the pattern is related to the axial broadening of its spatial frequencies (Fig. 2 c). Emission light from the sample is observed by a charge-coupled device (CCD) camera via a dichroic mirror (DM).
FIGURE 4
A cluster of red-fluorescent microspheres of nominal diameter 0.12 _μ_m, imaged with (a and c) conventional microscopy, and (b and d) structured illumination microscopy. (a and b) Single in-focus x,y sections illustrating the improvement of lateral resolution. (c and d) Single x,z sections, at the y position indicated by horizontal lines in panel a, illustrating removal of out-of-focus blur above and below the plane of focus. (e) Histograms of the apparent lateral and axial full width at half-maximum (FWHM) of individual green-fluorescent beads observed by three-dimensional SIM.
FIGURE 5
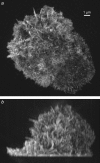
Maximum intensity projections through a three-dimensional structured illumination reconstruction of the actin cytoskeleton in an HL-60 cell, shown in top view (a) and side view (b). A video of this reconstruction is available on the journal web site (
Movie S1
).
FIGURE 6
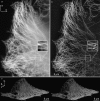
The microtubule cytoskeleton in HeLa cells. Axial maximum-intensity projections, using conventional microscopy (a) and structured-illumination microscopy (b), through a 244-nm-thick subset of the data (two sections). (Insets) two parallel microtubules spaced by 125 nm (arrows) are well resolved in the structured illumination reconstruction, but unresolved by conventional microscopy. The image contrast of the inset in a has been adjusted, for easier comparison. (c) Cross-eyed stereo view of projections through the structured-illumination reconstruction. The data value of each voxel controlled both the brightness and the opacity of that voxel in the rendering. A video of this reconstruction is available on the journal web site (
Movie S2
).
FIGURE 7
The synaptonemal complex (SC) in a maize meiocyte nucleus during the late zygotene stage of meiosis. Fixed meiocytes were immunofluorescently labeled for the axial/lateral element components AFD1 (red fluorescence, here displayed as magenta) and ASY1 (green) of the synaptonemal complex. Under the fixation conditions used, the ASY1 epitope is inaccessible to the antibodies when synapsis is complete; the synapsed regions therefore fluoresce only in red, whereas the unsynapsed regions fluoresce in both green and red. In fully synapsed chromosomes, the lateral elements align as two parallel strands separated by ∼170 nm. (a) Maximum-intensity projection through a three-dimensional structured-illumination reconstruction of an entire nucleus (data set thickness, 8 _μ_m). Separate renderings of the AFD1 and ASY1 channels of this image are available on the journal website (
Fig. S1
). (b) Transmission electron micrograph of a silver-stained chromosome-spread preparation of a maize pachytene meiocyte, showing the three elements of the SC surrounded by the paired chromosomes. (LE, lateral elements; CE, central element; Ch, chromatin.) (c) Magnification of the cyan-boxed region, maximum-intensity-projected through 1.5 _μ_m of the sample thickness, showing a transition from unpaired to paired SC (arrows). (d and e) Magnifications of the white-boxed region of the specimen, imaged with conventional microscopy (d) or structured illumination microscopy (e). Panels d and e show maximum-intensity projections of only three axial sections, representing 375 nm of sample thickness, to avoid unnecessary blur in the conventional image. The lateral elements in synapsed regions, which are unresolvable by conventional microscopy (d), are well resolved by structured illumination microscopy (a, c, and e), and the twisting of the two strands around each other can be clearly followed (e, compare with b). A video of this reconstruction is available on the journal web site (
Movie S3
).
Similar articles
- Practical structured illumination microscopy.
Rego EH, Shao L. Rego EH, et al. Methods Mol Biol. 2015;1251:175-92. doi: 10.1007/978-1-4939-2080-8_10. Methods Mol Biol. 2015. PMID: 25391800 - Transillumination spatially modulated illumination microscopy.
Pitris C, Eracleous P. Pitris C, et al. Opt Lett. 2005 Oct 1;30(19):2590-2. doi: 10.1364/ol.30.002590. Opt Lett. 2005. PMID: 16208909 - Limits for reduction of effective focal volume in multiple-beam light microscopy.
Arkhipov A, Schulten K. Arkhipov A, et al. Opt Express. 2009 Feb 16;17(4):2861-70. doi: 10.1364/oe.17.002861. Opt Express. 2009. PMID: 19219190 Free PMC article. - Recent advancements in structured-illumination microscopy toward live-cell imaging.
Hirano Y, Matsuda A, Hiraoka Y. Hirano Y, et al. Microscopy (Oxf). 2015 Aug;64(4):237-49. doi: 10.1093/jmicro/dfv034. Epub 2015 Jun 30. Microscopy (Oxf). 2015. PMID: 26133185 Review. - Widefield fluorescence microscopy with extended resolution.
Stemmer A, Beck M, Fiolka R. Stemmer A, et al. Histochem Cell Biol. 2008 Nov;130(5):807-17. doi: 10.1007/s00418-008-0506-8. Epub 2008 Sep 23. Histochem Cell Biol. 2008. PMID: 18810482 Review.
Cited by
- Interferometer-based structured-illumination microscopy utilizing complementary phase relationship through constructive and destructive image detection by two cameras.
Shao L, Winoto L, Agard DA, Gustafsson MG, Sedat JW. Shao L, et al. J Microsc. 2012 Jun;246(3):229-36. doi: 10.1111/j.1365-2818.2012.03604.x. Epub 2012 Apr 4. J Microsc. 2012. PMID: 22472010 Free PMC article. - The neuropilin 1 cytoplasmic domain is required for VEGF-A-dependent arteriogenesis.
Lanahan A, Zhang X, Fantin A, Zhuang Z, Rivera-Molina F, Speichinger K, Prahst C, Zhang J, Wang Y, Davis G, Toomre D, Ruhrberg C, Simons M. Lanahan A, et al. Dev Cell. 2013 Apr 29;25(2):156-68. doi: 10.1016/j.devcel.2013.03.019. Dev Cell. 2013. PMID: 23639442 Free PMC article. - Improving signal-to-noise ratio of structured light microscopy based on photon reassignment.
Singh VR, Choi H, Yew EY, Bhattacharya D, Yuan L, Sheppard CJ, Rajapakse JC, Barbastathis G, So PT. Singh VR, et al. Biomed Opt Express. 2012 Jan 1;3(1):206-14. doi: 10.1364/BOE.3.000206. Epub 2011 Dec 22. Biomed Opt Express. 2012. PMID: 22254180 Free PMC article. - Sub-synaptic, multiplexed analysis of proteins reveals Fragile X related protein 2 is mislocalized in Fmr1 KO synapses.
Wang GX, Smith SJ, Mourrain P. Wang GX, et al. Elife. 2016 Oct 22;5:e20560. doi: 10.7554/eLife.20560. Elife. 2016. PMID: 27770568 Free PMC article. - mMaple: a photoconvertible fluorescent protein for use in multiple imaging modalities.
McEvoy AL, Hoi H, Bates M, Platonova E, Cranfill PJ, Baird MA, Davidson MW, Ewers H, Liphardt J, Campbell RE. McEvoy AL, et al. PLoS One. 2012;7(12):e51314. doi: 10.1371/journal.pone.0051314. Epub 2012 Dec 11. PLoS One. 2012. PMID: 23240015 Free PMC article.
References
- Agard, D. A., Y. Hiraoka, P. Shaw, and J. W. Sedat. 1989. Fluorescence microscopy in three dimensions. Methods Cell Biol. 30:353–377. - PubMed
- Pawley, J. B. 2006. Handbook of Biological Confocal Microscopy. Plenum Press, New York.
- Diaspro, A. 2002. Confocal and Two-Photon Microscopy: Foundations, Applications, and Advances. Wiley-Liss, New York.
- Wilson, T. 1995. The role of the pinhole in confocal imaging system. In Handbook of Biological Confocal Microscopy. J. B. Pawley, editor. Plenum Press, New York.
- Gustafsson, M. G. L. 2000. Surpassing the lateral resolution limit by a factor of two using structured illumination microscopy. J. Microsc. 198:82–87. - PubMed
Publication types
MeSH terms
Grants and funding
- GM48547/GM/NIGMS NIH HHS/United States
- GM31627/GM/NIGMS NIH HHS/United States
- R01 GM025101/GM/NIGMS NIH HHS/United States
- R01 GM031627/GM/NIGMS NIH HHS/United States
- GM25101/GM/NIGMS NIH HHS/United States
- R01 GM048547/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous