THM1 negatively modulates mouse sonic hedgehog signal transduction and affects retrograde intraflagellar transport in cilia - PubMed (original) (raw)
. 2008 Apr;40(4):403-410.
doi: 10.1038/ng.105. Epub 2008 Mar 9.
Courtney J Haycraft # 2, Tatiana Y Besschetnova # 3, Annick Turbe-Doan 1, Rolf W Stottmann 1, Bruce J Herron 1, Allyson L Chesebro 1, Haiyan Qiu 1, Paul J Scherz 4, Jagesh V Shah 3, Bradley K Yoder 2, David R Beier 1
Affiliations
- PMID: 18327258
- PMCID: PMC4817720
- DOI: 10.1038/ng.105
THM1 negatively modulates mouse sonic hedgehog signal transduction and affects retrograde intraflagellar transport in cilia
Pamela V Tran et al. Nat Genet. 2008 Apr.
Abstract
Characterization of previously described intraflagellar transport (IFT) mouse mutants has led to the proposition that normal primary cilia are required for mammalian cells to respond to the sonic hedgehog (SHH) signal. Here we describe an N-ethyl-N-nitrosourea-induced mutant mouse, alien (aln), which has abnormal primary cilia and shows overactivation of the SHH pathway. The aln locus encodes a novel protein, THM1 (tetratricopeptide repeat-containing hedgehog modulator-1), which localizes to cilia. aln-mutant cilia have bulb-like structures at their tips in which IFT proteins (such as IFT88) are sequestered, characteristic of Chlamydomonas reinhardtii and Caenorhabditis elegans retrograde IFT mutants. RNA-interference knockdown of Ttc21b (which we call Thm1 and which encodes THM1) in mouse inner medullary collecting duct cells expressing an IFT88-enhanced yellow fluorescent protein fusion recapitulated the aln-mutant cilial phenotype, and live imaging of these cells revealed impaired retrograde IFT. In contrast to previously described IFT mutants, Smoothened and full-length glioblastoma (GLI) proteins localize to aln-mutant cilia. We hypothesize that the aln retrograde IFT defect causes sequestration of IFT proteins in aln-mutant cilia and leads to the overactivated SHH signaling phenotype. Specifically, the aln mutation uncouples the roles of anterograde and retrograde transport in SHH signaling, suggesting that anterograde IFT is required for GLI activation and that retrograde IFT modulates this event.
Figures
Figure 1
Ttc21b is mutated in _aln_-mutant mice. (a) An A→C mutation that converts a glutamine to a proline at residue 15 of THM1 was identified in the aln mutant. (b) Western blot analysis of THM1 on E10.5 wild-type (WT), heterozygous (het) and _aln_-mutant head extracts revealed an absence of THM1 protein in aln mutant extracts. (c) THM1 sequence encompassing the aln mutation is highly conserved across species. In the C. elegans and C. reinhardtii orthologs, the substitution of glutamine by arginine is predicted to be a structurally conservative amino acid substitution. The start methionine is shown for Mus musculus, Bos taurus, Homo sapiens and Gallus gallus orthologs. For the other species listed, the presumptive first exon is absent from the current annotations. Asterisks denote site of aln mutation.
Figure 2
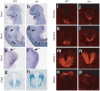
SHH signaling targets are misexpressed in aln mutants. (a–h) Whole-mount in situ hybridization for Ptch1 on E9.5 wild-type (WT) and _aln_-mutant embryos. In aln mutants, Ptch1 was expressed at greater intensities in the region of the maxilla and first branchial arches (brackets) and in the somites (arrowheads). Ptch1 was also expressed ectopically in the anterior region of the _aln_-mutant limb (arrows). (g–p) Analysis of dorsoventral patterning of the _aln_-mutant caudal neural tube at E9.5 using in situ hybridization (g,h) and immunofluorescence (i–p). Normal expression patterns were observed in wild-type mice. In contrast, the expression domains of ventral markers FOXA2, NKX2.2, NKX6.1 and Olig2 were expanded dorsally by the aln mutation, whereas dorsal marker MSX2 was shifted dorsally and confined to a smaller domain than in wild-type embryos, indicating that the _aln_-mutant neural tube is ventralized. Normal dorsoventral patterning was observed at rostral levels of the _aln_-mutant neural tube (data not shown).
Figure 3
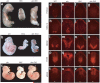
Genetic analyses of epistasis between mice mutant for aln and mice mutant for Shh, Smo or Gli2. (a) Frontal view of a Shh mutant at postnatal day 1.0 shows truncated limbs (asterisks). In contrast, limbs and certain head structures were partially restored in the aln Shh double mutant. (b) At E10.5, Smo mutants are arrested and unturned, with poor cranial development. In contrast, the aln Smo double-mutant embryo has partially turned and has more developed head structures. These double mutant phenotypes indicate that THM1 acts as a negative regulator downstream of both SHH and SMO. (c) At E10.5, Gli2 mutants have well-developed telencephalic vesicles, which is not the case in aln mutants. However, aln Gli2 double-mutant mice also show well-developed telencephalic vesicles, indicating that THM1 acts upstream of GLI2. Arrows point to a groove normally present caudal to the telencephalic vesicles, which is absent in aln mutants. (d–w) Dorsoventral patterning of the caudal spinal cord of E10.5 wild-type (WT), aln, Gli2 and aln Gli2 double-mutant embryos. Normal patterning is observed in wild-type embryos. In aln mutants, the neural tube is ventralized (see also Fig. 2j,l,n,p). In contrast, the aln Gli2 double-mutant spinal cord is dorsalized, resembling that of the Gli2 mutant; FOXA2 is absent and MNR2 is expanded to the most ventral regions of the spinal cord, whereas PAX6 and MSX2 are shifted ventrally. These analyses indicate that the ventralization of the _aln_-mutant caudal neural tube is largely mediated by GLI2, which is inappropriately activated in aln mutants.
Figure 4
GLI3 protein and transcript levels are elevated in aln mutants. (a) Western blot analysis of GLI3 in extracts of anterior (A) and posterior (P) limb buds of E10.5 wild-type (WT) and _aln_-mutant mice. _aln_-mutant anterior limb buds show increased levels of GLI3A and GLI3R proteins relative to wild type. (b) Whole-mount in situ hybridization for Gli3 on E10.5 embryos shows more intense expression in aln mutants, particularly in the branchial arches (brackets) and the limb (arrows). In the limb, the Gli3 expression domain appears less graded in aln mutants than in wild-type mice.
Figure 5
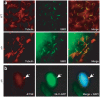
IFT88 is sequestered in _aln_-mutant primary cilia. (a) Immunofluorescence analysis of THM1 (green) and acetylated α-tubulin (red) in polarized IMCD cells and in nodal cilia of E8.0 embryos. THM1 colocalizes with acetylated α-tubulin in the cilial axoneme and is expressed in a punctate pattern from the base to the tip of the cilium. (b) Immunostaining for IFT88 (green) and acetylated α-tubulin (red) on cultured primary limb cells showed much more intense staining of IFT88 at the distal ends of _aln_-mutant cilia relative to wild type. Identical exposure times were used to image wild-type and _aln_-mutant cells. In aln mutants, IFT88 is visualized adjacent to, but not overlapping with, γ-tubulin, which stains the base of cilia (data not shown). Axonemes stained by acetylated α-tubulin also appeared shorter in aln mutants than in wild type. (c) Scanning electron microscopy analysis of wild-type and _aln_-mutant limb ectodermal primary cilia revealed bulb-like structures at the distal tips of _aln_-mutant cilia. Scale bar represents 1 μm.
Figure 6
SMO and GLI proteins translocate to _aln_-mutant cilia. (a) Whole-mount immunofluorescence analysis of SMO in wild-type (WT) and _aln_-mutant embryos showed colocalization of SMO (red) with acetylated α-tubulin (green) in nodal cilia. (b) Overexpression of GLI proteins in _aln_-mutant MEFs. _aln_-mutant MEFs were cultured in serum-free medium to promote the growth of cilia, then infected with adenoviruses expressing green fluorescent protein (GFP)-fused GLI1 and immunostained for IFT88. Full-length GLI1-GFP (green) fusion proteins co-localized with IFT88 (red), which stains the distal tips of _aln_-mutant cilia (see Fig. 5 and Supplementary Fig. 4).
Figure 7
Retrograde IFT is impaired in cilia of THM1-deficient IMCD cells. (a) Western blot analysis of IFT8-EYFP in wild-type (mIMCD) and m368-2 cell extracts. IFT88 and IFT88-EYFP bands represent endogenously and exogenously expressed protein, respectively. (b) Western blot analysis of THM1 in EV4, R1-4, R1-2 and R1-5 cell extracts. Expression of THM1 in R1-4, R1-2 and R1-5 cell lines was about 12.5%, 11.5% and 73% of that in control EV4 cells. (c) Anterograde and retrograde IFT velocities (mean ± s.d.) of IFT88-EYFP in cilia of EV4, R1-4, R1-2 and R1-5 cell lines. Retrograde IFT velocities are significantly lower in R1-4 and R1-2 cells (*, P < 0.002) than in EV4 and R1-5 cells. (d) Live-cell images of EV4 and R1-4 cell lines (top and bottom left panels) revealed punctate distribution of IFT88-EYFP from the base to the tip of cilia. R1-4 cell line showed a much higher accumulation of IFT88 at the tip of the cilium (white arrows, top panels). White arrows on bottom left panels indicate tips of cilia. Broken and solid black lines on both EV4 and R1-4 kymographs represent anterograde and retrograde IFT, respectively (bottom right panels). Scale bars, 5 μm.
Similar articles
- Genetic interaction of mammalian IFT-A paralogs regulates cilia disassembly, ciliary entry of membrane protein, Hedgehog signaling, and embryogenesis.
Wang W, Allard BA, Pottorf TS, Wang HH, Vivian JL, Tran PV. Wang W, et al. FASEB J. 2020 May;34(5):6369-6381. doi: 10.1096/fj.201902611R. Epub 2020 Mar 13. FASEB J. 2020. PMID: 32167205 Free PMC article. - Cilia and Hedgehog responsiveness in the mouse.
Huangfu D, Anderson KV. Huangfu D, et al. Proc Natl Acad Sci U S A. 2005 Aug 9;102(32):11325-30. doi: 10.1073/pnas.0505328102. Epub 2005 Aug 1. Proc Natl Acad Sci U S A. 2005. PMID: 16061793 Free PMC article. - Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors.
Liu A, Wang B, Niswander LA. Liu A, et al. Development. 2005 Jul;132(13):3103-11. doi: 10.1242/dev.01894. Epub 2005 Jun 1. Development. 2005. PMID: 15930098 - Gli proteins in development and disease.
Hui CC, Angers S. Hui CC, et al. Annu Rev Cell Dev Biol. 2011;27:513-37. doi: 10.1146/annurev-cellbio-092910-154048. Epub 2011 Jul 21. Annu Rev Cell Dev Biol. 2011. PMID: 21801010 Review. - The emergent design of the neural tube: prepattern, SHH morphogen and GLI code.
Ruiz i Altaba A, Nguyên V, Palma V. Ruiz i Altaba A, et al. Curr Opin Genet Dev. 2003 Oct;13(5):513-21. doi: 10.1016/j.gde.2003.08.005. Curr Opin Genet Dev. 2003. PMID: 14550418 Review.
Cited by
- Mutations in mouse Ift144 model the craniofacial, limb and rib defects in skeletal ciliopathies.
Ashe A, Butterfield NC, Town L, Courtney AD, Cooper AN, Ferguson C, Barry R, Olsson F, Liem KF Jr, Parton RG, Wainwright BJ, Anderson KV, Whitelaw E, Wicking C. Ashe A, et al. Hum Mol Genet. 2012 Apr 15;21(8):1808-23. doi: 10.1093/hmg/ddr613. Epub 2012 Jan 6. Hum Mol Genet. 2012. PMID: 22228095 Free PMC article. - Architecture and function of IFT complex proteins in ciliogenesis.
Taschner M, Bhogaraju S, Lorentzen E. Taschner M, et al. Differentiation. 2012 Feb;83(2):S12-22. doi: 10.1016/j.diff.2011.11.001. Epub 2011 Nov 25. Differentiation. 2012. PMID: 22118932 Free PMC article. Review. - Mutations in Dnaaf1 and Lrrc48 Cause Hydrocephalus, Laterality Defects, and Sinusitis in Mice.
Ha S, Lindsay AM, Timms AE, Beier DR. Ha S, et al. G3 (Bethesda). 2016 Aug 9;6(8):2479-87. doi: 10.1534/g3.116.030791. G3 (Bethesda). 2016. PMID: 27261005 Free PMC article. - Thm2 interacts with paralog, Thm1, and sensitizes to Hedgehog signaling in postnatal skeletogenesis.
Allard BA, Wang W, Pottorf TS, Mumtaz H, Jack BM, Wang HH, Silva LM, Jacobs DT, Wang J, Bumann EE, Tran PV. Allard BA, et al. Cell Mol Life Sci. 2021 Apr;78(7):3743-3762. doi: 10.1007/s00018-021-03806-w. Epub 2021 Mar 8. Cell Mol Life Sci. 2021. PMID: 33683377 Free PMC article. - Scoring a backstage pass: mechanisms of ciliogenesis and ciliary access.
Garcia-Gonzalo FR, Reiter JF. Garcia-Gonzalo FR, et al. J Cell Biol. 2012 Jun 11;197(6):697-709. doi: 10.1083/jcb.201111146. J Cell Biol. 2012. PMID: 22689651 Free PMC article. Review.
References
- Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–3087. - PubMed
- Wang B, Fallon JF, Beachy PA. Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell. 2000;100:423–434. - PubMed
- Liu A, Wang B, Niswander LA. Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development. 2005;132:3103–3111. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HD036404/HD/NICHD NIH HHS/United States
- P50 DK074030-019001/DK/NIDDK NIH HHS/United States
- P50 DK074030/DK/NIDDK NIH HHS/United States
- P30 DK074038/DK/NIDDK NIH HHS/United States
- R01HD36404/HD/NICHD NIH HHS/United States
- F32 HD053198-01A2/HD/NICHD NIH HHS/United States
- P50 DK074030-029001/DK/NIDDK NIH HHS/United States
- F32 HD053198/HD/NICHD NIH HHS/United States
- R01HD056030/HD/NICHD NIH HHS/United States
- R01 HD056030/HD/NICHD NIH HHS/United States
- F32 HD053198-02/HD/NICHD NIH HHS/United States
- P50DK074030/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous