Subversion of actin dynamics by EspM effectors of attaching and effacing bacterial pathogens - PubMed (original) (raw)
Subversion of actin dynamics by EspM effectors of attaching and effacing bacterial pathogens
Ana Arbeloa et al. Cell Microbiol. 2008 Jul.
Abstract
Rho GTPases are common targets of bacterial toxins and type III secretion system effectors. IpgB1 and IpgB2 of Shigella and Map of enteropathogenic (EPEC) and enterohemorrhagic (EHEC) Escherichia coli were recently grouped together on the basis that they share a conserved WxxxE motif. In this study, we characterized six WxxxE effectors from attaching and effacing pathogens: TrcA and EspM1 of EPEC strain B171, EspM1 and EspM2 of EHEC strain Sakai and EspM2 and EspM3 of Citrobacter rodentium. We show that EspM2 triggers formation of global parallel stress fibres, TrcA and EspM1 induce formation of localized parallel stress fibres and EspM3 triggers formation of localized radial stress fibres. Using EspM2 and EspM3 as model effectors, we report that while substituting the conserved Trp with Ala abolished activity, conservative Trp to Tyr or Glu to Asp substitutions did not affect stress-fibre formation. We show, using dominant negative constructs and chemical inhibitors, that the activity of EspM2 and EspM3 is RhoA and ROCK-dependent. Using Rhotekin pull-downs, we have shown that EspM2 and EspM3 activate RhoA; translocation of EspM2 and EspM3 triggered phosphorylation of cofilin. These results suggest that the EspM effectors modulate actin dynamics by activating the RhoA signalling pathway.
Figures
Fig. 1
The EspM WxxxE effector proteins in A/E pathogen group. A. Multiple sequence alignment with hierarchical clustering of S. exneri IpgB2, EspM1 and EspM2 from EHEC O157:H7 Sakai, EspM2 and EspM3 from C. rodentium (CR) and TrcA and EspM1 from EPEC B171. Similar and identical residues are highlighted in bright and dark grey respectively. The conserved motif WxxxE is boxed. Conserved residues subjected to mutagenesis in this study are tagged with a star. B. Radial phylogeny tree showing the subfamily of IpgB2-like WxxxE effectors in the A/E group, EPEC E2348/69 Map and Shigella IpgB1 and IpgB2.
Fig. 2
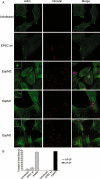
The EspM A/E effectors trigger formation of actin stress fibres in infected Swiss 3T3 cells. A. Fluorescence microscopy of uninfected Swiss 3T3 cell or cells infected for 90 min with wild-type E2348/69 and E2348/69 expressing EspM2 (Sakai), EspM1 (B171) and EspM3 (CR). Actin was stained with Oregon green phalloidin, EPEC were detected with rabbit anti-0127 antibody (merged column) and vinculin was visualised using a monoclonal mouse antibody. GP-SF, LP-SF and LR-SF were observed in cell infected with EPEC expressing EspM2, EspM1 and EspM3 respectively. All the recombinant strains also induced formation of actin pedestals (insets). B. Quantification of parallel and radial stress-fibre formation in uninfected Swiss cells or after 90 min infection with wild-type E2348/69 and E2348/69 expressing EspM2 and EspM3. One hundred cells were counted in triplicate in three independent experiments. Results are presented as mean ± SEM.
Fig. 3
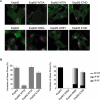
Effect of site-directed mutagenesis on stress-fibre formation. A. Merged fluorescence microscopy of Swiss 3T3 cells infected for 90 min with primed E2348/69 expressing EspM2 (Sakai) and EspM3 (CR) derivative mutants within the conserved WxxxE motif. Actin was stained with Oregon green phalloidin and EPEC were detected with rabbit anti-0127 antibody. B. Quantification of stress-fibre formation in Swiss cells after 90 min infection. One hundred infected cells were counted in triplicate from three independent experiments. Results are presented as mean ± SEM.
Fig. 4
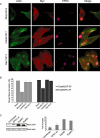
Stress-fibre formation by EspM2 and EspM3 is RhoA-dependent. A. Swiss 3T3 cells were transfected with dominant negative GTPase contracts 24 h prior to infection with E2348/69 expressing EspM2. Actin was stained with Oregon green phalloidin, the Myc-tagged GTPases with mouse anti-myc and EPEC with rabbit anti-O127. Actin-rich pedestals are observed under adherent bacteria in all transfected cells. GP-SF are observed, at the level of mock transfection, in cells transfected with dominant negatives Cdc42N17 or Rac1N17. In contrast, transfection with RhoN19 inhibited formation of GP-SF. B. Quantification of stress-fibre formation in transfected Swiss 3T3 cells infected for 90 min with E2348/69 expressing EspM2 or EspM3. Formation of stress fibres in one hundred transfected infected cells were counted in triplicate from three independent experiments. Results are presented as mean ± SEM. Formation of stress fibres depending on EspM2 or EspM3 is affected by expression of RhoAN19, but not by expression of Cdc42N17 or RacN17. C. Swiss 3T3 cells were infected with wild-type E2348/69 or E2348/69 expressing EspM2 or EspM3. Cells were lysed and a GST fusion of the Rho binding domain of Rhotekin was used to co-purify RhoA-GTP. Total RhoA in the lysates and RhoA-GTP were detected by Western blot with anti-RhoA antibodies. Quantified data are means ± SD of the results of three independent experiments.
Fig. 5
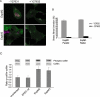
EspM2 and EspM3 trigger the ROCK-LIMK-cofilin pathway. A. Fluorescence microscopy of Swiss 3T3 cells incubated for 1 h in presence or absence of 10 μM of the ROCK inhibitor Y-27632 and infected for 90 min with E2348/69 expressing EspM2 or EspM3. Y-27632 did not affect formation of actin-rich pedestals, but abolished formation of stress fibres. B. Percentage of Swiss 3T3 cells presenting stress fibres in the presence or absence of Y-27632. One hundred cells were counted in triplicate from three independent experiments. Results are presented as mean ± SEM. C. Swiss 3T3 cells were infected for 90 min with wild-type E2348/69 or E2348/69 expressing EspM2 or EspM3. The levels of cofilin and phospho-cofilin were determined by Western blot using anti-cofilin and anti-phospho-cofilin antibodies. Quantification of densitometry was conducted using image J software and results presented here represent the mean ratio ± SD of four independent experiments.
Similar articles
- Bacterial guanine nucleotide exchange factors SopE-like and WxxxE effectors.
Bulgin R, Raymond B, Garnett JA, Frankel G, Crepin VF, Berger CN, Arbeloa A. Bulgin R, et al. Infect Immun. 2010 Apr;78(4):1417-25. doi: 10.1128/IAI.01250-09. Epub 2010 Feb 1. Infect Immun. 2010. PMID: 20123714 Free PMC article. Review. - EspO1-2 regulates EspM2-mediated RhoA activity to stabilize formation of focal adhesions in enterohemorrhagic Escherichia coli-infected host cells.
Morita-Ishihara T, Miura M, Iyoda S, Izumiya H, Watanabe H, Ohnishi M, Terajima J. Morita-Ishihara T, et al. PLoS One. 2013;8(2):e55960. doi: 10.1371/journal.pone.0055960. Epub 2013 Feb 8. PLoS One. 2013. PMID: 23409096 Free PMC article. - EspT triggers formation of lamellipodia and membrane ruffles through activation of Rac-1 and Cdc42.
Bulgin RR, Arbeloa A, Chung JC, Frankel G. Bulgin RR, et al. Cell Microbiol. 2009 Feb;11(2):217-29. doi: 10.1111/j.1462-5822.2008.01248.x. Epub 2008 Oct 30. Cell Microbiol. 2009. PMID: 19016787 Free PMC article. - EspM2 is a RhoA guanine nucleotide exchange factor.
Arbeloa A, Garnett J, Lillington J, Bulgin RR, Berger CN, Lea SM, Matthews S, Frankel G. Arbeloa A, et al. Cell Microbiol. 2010 May 1;12(5):654-64. doi: 10.1111/j.1462-5822.2009.01423.x. Epub 2009 Dec 21. Cell Microbiol. 2010. PMID: 20039879 Free PMC article. - Cytoskeletal mechanisms regulating attaching/effacing bacteria interactions with host cells: It takes a village to build the pedestal.
Naydenov NG, Marino-Melendez A, Campellone KG, Ivanov AI. Naydenov NG, et al. Bioessays. 2024 Nov;46(11):e2400160. doi: 10.1002/bies.202400160. Epub 2024 Sep 20. Bioessays. 2024. PMID: 39301984 Review.
Cited by
- EspH interacts with the host active Bcr related (ABR) protein to suppress RhoGTPases.
Ramachandran RP, Nandi I, Haritan N, Zlotkin-Rivkin E, Keren Y, Danieli T, Lebendiker M, Melamed-Book N, Breuer W, Reichmann D, Aroeti B. Ramachandran RP, et al. Gut Microbes. 2022 Jan-Dec;14(1):2130657. doi: 10.1080/19490976.2022.2130657. Gut Microbes. 2022. PMID: 36219160 Free PMC article. - Transposon-insertion sequencing screens unveil requirements for EHEC growth and intestinal colonization.
Warr AR, Hubbard TP, Munera D, Blondel CJ, Abel Zur Wiesch P, Abel S, Wang X, Davis BM, Waldor MK. Warr AR, et al. PLoS Pathog. 2019 Aug 12;15(8):e1007652. doi: 10.1371/journal.ppat.1007652. eCollection 2019 Aug. PLoS Pathog. 2019. PMID: 31404118 Free PMC article. - Bacteria and host interactions in the gut epithelial barrier.
Ashida H, Ogawa M, Kim M, Mimuro H, Sasakawa C. Ashida H, et al. Nat Chem Biol. 2011 Dec 15;8(1):36-45. doi: 10.1038/nchembio.741. Nat Chem Biol. 2011. PMID: 22173358 Review. - VopX, a novel Vibrio cholerae T3SS effector, modulates host actin dynamics.
Ulbrich M, Seward CH, Ivanov AI, Ward BM, Butler JS, Dziejman M. Ulbrich M, et al. mBio. 2025 Mar 12;16(3):e0301824. doi: 10.1128/mbio.03018-24. Epub 2025 Jan 29. mBio. 2025. PMID: 39878476 Free PMC article. - Distribution of espM and espT among enteropathogenic and enterohaemorrhagic Escherichia coli.
Arbeloa A, Blanco M, Moreira FC, Bulgin R, López C, Dahbi G, Blanco JE, Mora A, Alonso MP, Mamani RC, Gomes TAT, Blanco J, Frankel G. Arbeloa A, et al. J Med Microbiol. 2009 Aug;58(Pt 8):988-995. doi: 10.1099/jmm.0.010231-0. Epub 2009 Jun 15. J Med Microbiol. 2009. PMID: 19528152 Free PMC article.
References
- Alto NM, Shao F, Lazar CS, Brost RL, Chua G, Mattoo S, et al. Identification of a bacterial type III effector family with G protein mimicry functions. Cell. 2006;124:133–145. - PubMed
- Barthold SW, Coleman GL, Bhatt PN, Osbaldiston GW, Jonas AM. The etiology of transmissible murine colonic hyperplasia. Lab Anim Sci. 1976;26:889–894. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources