Increased mechanosensitivity and nuclear stiffness in Hutchinson-Gilford progeria cells: effects of farnesyltransferase inhibitors - PubMed (original) (raw)
Increased mechanosensitivity and nuclear stiffness in Hutchinson-Gilford progeria cells: effects of farnesyltransferase inhibitors
Valerie L R M Verstraeten et al. Aging Cell. 2008 Jun.
Abstract
Hutchinson-Gilford progeria syndrome (HGPS), reportedly a model for normal aging, is a genetic disorder in children marked by dramatic signs suggestive for premature aging. It is usually caused by de novo mutations in the nuclear envelope protein lamin A. Lamins are essential to maintaining nuclear integrity, and loss of lamin A/C results in increased cellular sensitivity to mechanical strain and defective mechanotransduction signaling. Since increased mechanical sensitivity in vascular cells could contribute to loss of smooth muscle cells and the development of arteriosclerosis--the leading cause of death in HGPS patients--we investigated the effect of mechanical stress on cells from HGPS patients. We found that skin fibroblasts from HGPS patients developed progressively stiffer nuclei with increasing passage number. Importantly, fibroblasts from HGPS patients had decreased viability and increased apoptosis under repetitive mechanical strain, as well as attenuated wound healing, and these defects preceded changes in nuclear stiffness. Treating fibroblasts with farnesyltransferase inhibitors restored nuclear stiffness in HGPS cells and accelerated the wound healing response in HGPS and healthy control cells by increasing the directional persistence of migrating cells. However, farnesyltransferase inhibitors did not improve cellular sensitivity to mechanical strain. These data suggest that increased mechanical sensitivity in HGPS cells is unrelated to changes in nuclear stiffness and that increased biomechanical sensitivity could provide a potential mechanism for the progressive loss of vascular smooth muscle cells under physiological strain in HGPS patients.
Figures
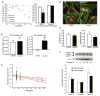
Figure 1. Increased nuclear stiffness in late-passage HGPS fibroblasts
(A) Nuclear shape was analyzed in HGPS fibroblasts and control cells at a wide range of passages. Late passage HGPS had significantly fewer normally shaped nuclei than early passage HGPS cells and passage matched controls (**, P < 0.01 vs. early passage HGPS cells and late passage controls) (B) Nuclear stiffness in early and late passage HGPS cells and healthy control cells was evaluated by cellular strain application. The extent of induced nuclear deformations was expressed as a ratio of nuclear strain to applied membrane strain (normalized nuclear strain). Lower values indicate increased nuclear stiffness. (C) Increase in nuclear stiffness with increasing passage number seen in one representative cell strain (AG01972). The solid line depicts the linear regression, with dashed lines marking the 95% confidence interval. The slope was −8.8 ± 2.3 × 10−3/passage. (D) Fluorescence labeling of F-actin (green) and microtubules (red) indicating normal cytoskeletal structure in HGPS cells. Scale bar 20μm. (E) Cytoskeletal stiffness measured by magnetic bead microrheology. Bead displacement amplitude (left) and residual displacement (right) after sinusoidal force application were normal in HGPS cells. (F) Western analysis of lamin A, lamin C, and progerin of one representative HGPS cell line at various passages (top). Protein levels normalized to actin loading controls compared between early and late passage cells from four independent HGPS cell lines analyzed by densitometry (bottom).
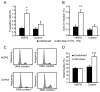
Figure 2. HGPS cells are more sensitive to mechanical strain
(A) HGPS fibroblasts subjected to 24h of repetitive, biaxial strain had increased fractions of PI-positive dead cells (*: P < 0.05 vs. unstrained controls). (B) Fraction of apoptotic (sub-G1) cells from the same experiments (**: P < 0.01 vs. unstrained controls). (C) Representative results of flow cytometry DNA content analysis of HGPS and control fibroblasts subjected to 24h of cyclic, biaxial strain and unstrained controls. (D) HGPS cells lacked the strain-induced cell proliferation seen in healthy control fibroblasts (***: P < 0.001 vs. unstrained controls).
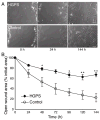
Figure 3. Impaired wound healing in HGPS fibroblasts
(A) Representative images of the wound area at 0, 24h, and 144h after wound creation for HGPS cells and healthy controls show the slower wound closure in HGPS cells. Scale bar 100μm. (B) The mean open wound area as a percentage of the initial wound area at different time points indicates a significantly delayed wound closure for HGPS fibroblasts compared to healthy controls (*: P < 0.05 vs. controls; **: P < 0.01 vs. controls).
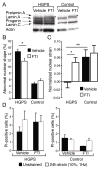
Figure 4. FTI treatment improved nuclear mechanics in HGPS cells
(A) Efficacy of FTI treatment was confirmed by Western analysis of total cell lysates as described previously (Toth et al. 2005). FTI treatment resulted in accumulation of prelamin A in the FTI-treated HGPS and control samples (asterisk). (B) FTI treatment reduced the frequency of abnormally shaped nuclei in HGPS cells (*: P < 0.05 vs. vehicle-treated cells). (C) FTI treatment restored nuclear stiffness in HGPS cells but had no effect on healthy control fibroblasts (*: P < 0.05; **: P < 0.01). (D) FTI-treatment had no effect on the fraction of dead (PI-positive) cells in response to 24h of cyclic, biaxial strain application (10% strain at 1Hz).
Figure 5. FTI treatment improved wound healing in HGPS and healthy control cells
(A) Representative images of the wound area at 0, 24h, and 144h after wound creation for HGPS cells treated with FTI or vehicle alone. Scale bar 100μm. (B) Left, representative examples of the residual open area for HGPS (filled symbols) and healthy control cells (open symbols) treated with FTI (triangle) or vehicle alone (circle) indicate that FTI treatment accelerates wound closure. Right, comparison of the residual open area after 24h and 144h for HGPS and healthy control cells treated with FTI or vehicle alone (*: P < 0.05; **: P < 0.01; ***: P < 0.001). (C) Wound healing experiments on wild-type (Lmna+/+, left), Lamin C–only (_Lmna_LCO/LCO, center), and lamin A/C–deficient (_Lmna_−/−, right) mouse embryo fibroblasts treated with 10μM FTI or vehicle alone (*: P < 0.05; **: P < 0.01; ***: P < 0.001). (D) BrdU-labeling revealed increased DNA synthesis at the wound edge within the first 24h after wounding. Scale bar 200μm. (E) Left, HGPS cells had significantly lower migration speeds compared to healthy control fibroblasts (**: P < 0.01). FTI treatment had no effect on migration speed. Right, FTI treatment increased the directional persistence time of migration in both HGPS and control fibroblasts.
Similar articles
- Identification of mitochondrial dysfunction in Hutchinson-Gilford progeria syndrome through use of stable isotope labeling with amino acids in cell culture.
Rivera-Torres J, Acín-Perez R, Cabezas-Sánchez P, Osorio FG, Gonzalez-Gómez C, Megias D, Cámara C, López-Otín C, Enríquez JA, Luque-García JL, Andrés V. Rivera-Torres J, et al. J Proteomics. 2013 Oct 8;91:466-77. doi: 10.1016/j.jprot.2013.08.008. Epub 2013 Aug 20. J Proteomics. 2013. PMID: 23969228 - Incomplete processing of mutant lamin A in Hutchinson-Gilford progeria leads to nuclear abnormalities, which are reversed by farnesyltransferase inhibition.
Glynn MW, Glover TW. Glynn MW, et al. Hum Mol Genet. 2005 Oct 15;14(20):2959-69. doi: 10.1093/hmg/ddi326. Epub 2005 Aug 26. Hum Mol Genet. 2005. PMID: 16126733 - Hutchinson-Gilford progeria mutant lamin A primarily targets human vascular cells as detected by an anti-Lamin A G608G antibody.
McClintock D, Gordon LB, Djabali K. McClintock D, et al. Proc Natl Acad Sci U S A. 2006 Feb 14;103(7):2154-9. doi: 10.1073/pnas.0511133103. Epub 2006 Feb 6. Proc Natl Acad Sci U S A. 2006. PMID: 16461887 Free PMC article. - Vascular smooth muscle cell loss underpins the accelerated atherosclerosis in Hutchinson-Gilford progeria syndrome.
Hamczyk MR, Andrés V. Hamczyk MR, et al. Nucleus. 2019 Dec;10(1):28-34. doi: 10.1080/19491034.2019.1589359. Nucleus. 2019. PMID: 30900948 Free PMC article. Review. - Hutchinson-Gilford progeria syndrome as a model for vascular aging.
Brassard JA, Fekete N, Garnier A, Hoesli CA. Brassard JA, et al. Biogerontology. 2016 Feb;17(1):129-45. doi: 10.1007/s10522-015-9602-z. Epub 2015 Sep 2. Biogerontology. 2016. PMID: 26330290 Review.
Cited by
- The intrinsic stiffness of human trabecular meshwork cells increases with senescence.
Morgan JT, Raghunathan VK, Chang YR, Murphy CJ, Russell P. Morgan JT, et al. Oncotarget. 2015 Jun 20;6(17):15362-74. doi: 10.18632/oncotarget.3798. Oncotarget. 2015. PMID: 25915531 Free PMC article. - Sensing the squeeze: nuclear mechanotransduction in health and disease.
Srivastava LK, Ehrlicher AJ. Srivastava LK, et al. Nucleus. 2024 Dec;15(1):2374854. doi: 10.1080/19491034.2024.2374854. Epub 2024 Jul 1. Nucleus. 2024. PMID: 38951951 Free PMC article. Review. - Measuring nucleus mechanics within a living multicellular organism: Physical decoupling and attenuated recovery rate are physiological protective mechanisms of the cell nucleus under high mechanical load.
Zuela-Sopilniak N, Bar-Sela D, Charar C, Wintner O, Gruenbaum Y, Buxboim A. Zuela-Sopilniak N, et al. Mol Biol Cell. 2020 Aug 1;31(17):1943-1950. doi: 10.1091/mbc.E20-01-0085. Epub 2020 Jun 17. Mol Biol Cell. 2020. PMID: 32583745 Free PMC article. - Mechanotransduction: from the cell surface to the nucleus via RhoA.
Burridge K, Monaghan-Benson E, Graham DM. Burridge K, et al. Philos Trans R Soc Lond B Biol Sci. 2019 Aug 19;374(1779):20180229. doi: 10.1098/rstb.2018.0229. Epub 2019 Jul 1. Philos Trans R Soc Lond B Biol Sci. 2019. PMID: 31431179 Free PMC article. Review. - Nuclear mechanics in disease.
Zwerger M, Ho CY, Lammerding J. Zwerger M, et al. Annu Rev Biomed Eng. 2011 Aug 15;13:397-428. doi: 10.1146/annurev-bioeng-071910-124736. Annu Rev Biomed Eng. 2011. PMID: 21756143 Free PMC article. Review.
References
- Al-Shali KZ, Hegele RA. Laminopathies and atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:1591–1595. - PubMed
- Baker PB, Baba N, Boesel CP. Cardiovascular abnormalities in progeria. Case report and review of the literature. Arch Pathol Lab Med. 1981;105:384–386. - PubMed
- Broers JL, Peeters EA, Kuijpers HJ, Endert J, Bouten CV, Oomens CW, Baaijens FP, Ramaekers FC. Decreased mechanical stiffness in LMNA−/− cells is caused by defective nucleo-cytoskeletal integrity: implications for the development of laminopathies. Hum Mol Genet. 2004;13:2567–2580. - PubMed
- Broers JL, Ramaekers FC, Bonne G, Yaou RB, Hutchison CJ. Nuclear lamins: laminopathies and their role in premature ageing. Physiol Rev. 2006;86:967–1008. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL082792-01A1/HL/NHLBI NIH HHS/United States
- R01 NS059348-02/NS/NINDS NIH HHS/United States
- R01 NS059348/NS/NINDS NIH HHS/United States
- HL079862/HL/NHLBI NIH HHS/United States
- R01 HL082792-03/HL/NHLBI NIH HHS/United States
- F32 HL079862/HL/NHLBI NIH HHS/United States
- HL082792/HL/NHLBI NIH HHS/United States
- R01 HL082792-02/HL/NHLBI NIH HHS/United States
- NS059348/NS/NINDS NIH HHS/United States
- R01 NS059348-01/NS/NINDS NIH HHS/United States
- R01 HL082792/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources