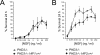Abnormal sympathoadrenal development and systemic hypotension in PHD3-/- mice - PubMed (original) (raw)
. 2008 May;28(10):3386-400.
doi: 10.1128/MCB.02041-07. Epub 2008 Mar 10.
Denis Gallagher, Alberto Pascual, Craig A Lygate, Joseph P de Bono, Lynn G Nicholls, Patricia Ortega-Saenz, Henrik Oster, Bhathiya Wijeyekoon, Andrew I Sutherland, Alexandra Grosfeld, Julian Aragones, Martin Schneider, Katie van Geyte, Dania Teixeira, Antonio Diez-Juan, Jose Lopez-Barneo, Keith M Channon, Patrick H Maxwell, Christopher W Pugh, Alun M Davies, Peter Carmeliet, Peter J Ratcliffe
Affiliations
- PMID: 18332118
- PMCID: PMC2423159
- DOI: 10.1128/MCB.02041-07
Abnormal sympathoadrenal development and systemic hypotension in PHD3-/- mice
Tammie Bishop et al. Mol Cell Biol. 2008 May.
Abstract
Cell culture studies have implicated the oxygen-sensitive hypoxia-inducible factor (HIF) prolyl hydroxylase PHD3 in the regulation of neuronal apoptosis. To better understand this function in vivo, we have created PHD3(-/-) mice and analyzed the neuronal phenotype. Reduced apoptosis in superior cervical ganglion (SCG) neurons cultured from PHD3(-/-) mice is associated with an increase in the number of cells in the SCG, as well as in the adrenal medulla and carotid body. Genetic analysis by intercrossing PHD3(-/-) mice with HIF-1a(+/-) and HIF-2a(+/-) mice demonstrated an interaction with HIF-2alpha but not HIF-1alpha, supporting the nonredundant involvement of a PHD3-HIF-2alpha pathway in the regulation of sympathoadrenal development. Despite the increased number of cells, the sympathoadrenal system appeared hypofunctional in PHD3(-/-) mice, with reduced target tissue innervation, adrenal medullary secretory capacity, sympathoadrenal responses, and systemic blood pressure. These observations suggest that the role of PHD3 in sympathoadrenal development extends beyond simple control of cell survival and organ mass, with functional PHD3 being required for proper anatomical and physiological integrity of the system. Perturbation of this interface between developmental and adaptive signaling by hypoxic, metabolic, or other stresses could have important effects on key sympathoadrenal functions, such as blood pressure regulation.
Figures
FIG. 1.
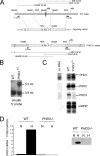
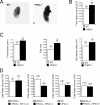
PHD3 targeting strategy. (A) Targeting strategy for PHD3 inactivation. Top: wild-type PHD3 allele diagram, indicating the position of exon 1 (dark box in the genomic structure). Middle: outline of the targeting vector, specifying the genomic sequences used as 5′ and 3′ homology flanks, inserted on each side of a neomycin resistance (Neo) cassette. A thymidine kinase (TK) gene outside the flanking homologies allowed for negative selection against random integration events. Bottom: replacement of exon 1 by the Neo cassette after homologous recombination. Diagnostic restriction fragments are indicated with their relative sizes by the thin lines under or above the alleles. Dark bars under the genes represent the probes used for Southern blot analysis. (B) Southern blot analysis of genomic DNA from recombinant ES cells, digested with HindIII and hybridized with the 5′ external probe. The 3.8-kb and 5.3-kb genomic fragments correspond to PHD3 wild-type (WT) and PHD3null alleles, respectively. (C) RNase protection assay demonstrating PHD1, -2, and -3 mRNA levels in total RNA extracted from wild-type and PHD3 −/− embryos. U6 small nuclear RNA (snRNP) was used as an internal control. (D) Quantitative RT-PCR (left panel) and Western blot assay (right panel) showing induction of PHD3 mRNA and protein in wild-type, but not PHD3 −/−, mouse embryonic fibroblasts in response to hypoxia. N, normoxia; H, hypoxia (1% oxygen for 16 h).
FIG. 2.
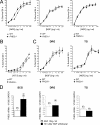
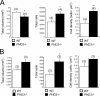
Survival of neurons cultured from PHD3 −/− mice. (A) NGF dose-response curves, demonstrating increased neuronal survival in neurons cultured from the SCG of P0 PHD3 −/− mice but not PHD1 −/− or PHD2 +/− mice. Neuronal survival was estimated by expressing the number of surviving neurons after 24 h in culture as a percentage of the initial number of neurons at 3 h postplating. (B) NT-3 dose-response curve, showing increased survival in neurons cultured from the SCG of P0 PHD3 −/− mice. (C) NGF dose-response curves, showing no change in survival in neurons cultured from the DRG and TG of P0 PHD3 −/− mice. (D) Quantitative RT-PCR showing induction of PHD3 mRNA after NGF withdrawal in the SCG, but not the DRG or TG, from P0 wild-type mice. Values for this and all subsequent figures are presented as means ± SEM (n = 3) for dose-response curves, or n as indicated in parentheses. *, P < 0.05 versus control; **, P < 0.01 versus control.
FIG. 3.
HIF dependence of the PHD3 survival effect. NGF dose-response curves for neonatal SCG neurons showing no difference in survival of neurons derived from PHD3 −/−; HIF-1_α+/− versus PHD3 −/− mice (A) and reduced survival of neurons derived from PHD3 −/−; HIF-2_α+/− versus PHD3 −/− mice (B).
FIG. 4.
Neurite length and arborization of sympathetic neurons cultured from PHD3 −/− mice. Increased neurite length and arborization in P0 PHD3 −/− mice at 0.4 (B) and 0.08 ng/ml NGF (C), but not at 10 ng/ml NGF (A). Neurite length is presented as the mean ± SEM on 40 to 70 neurons per genotype (similar results were obtained in three independent experiments). (D) Representative images of neurons cultured in 0.4 ng/ml NGF and fluorescently labeled with the vital dye calcein-AM. Bar, 50 μm.
FIG. 5.
Effect of genetic inactivation of PHD3 on anatomy of SCG. (A) Bright-field images of wild-type and PHD3 −/− neonatal SCG. Bar, 100 μm. (B) Neuronal complement of neonatal SCG in wild-type and PHD3 −/− mice; counts are of viable trypsin-dissociated neurons. (C) Stereological analysis of TH-positive neurons showing increased cell numbers in the SCG from adult PHD3 −/− mice. (D) Comparison of neuronal complement of neonatal SCG in mice of the indicated genotypes. Counts are as for panel B. HIF-2α heterozygosity, but not HIF-1α heterozygosity, is associated with reduced neuronal complement.
FIG. 6.
Effect of genetic inactivation of PHD3 on the anatomy of other sympathoadrenal tissues, the adrenal medulla (A) and carotid body (B). Stereological analysis of TH-positive neurosecretory cells demonstrating increased cell numbers in the adrenal medulla (A) and carotid body (B) of adult PHD3 −/− mice.
FIG. 7.
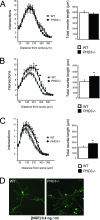
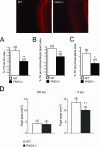
Sympathetic innervation of SCG target tissues from PHD3 −/− mice: immunohistochemistry demonstrating TH-positive neurons in SCG target tissues. Shown are representative images of TH-stained (bright red) neurons in the iris. Measured as the ratio of the TH-positively stained area over the total area of the SCG target tissue, there was decreased sympathetic innervation density of the iris (A), submandibular gland (B), and pineal gland (C). (D) Average pupil sizes in conscious, adult PHD3 −/− mice and wild-type controls under normal illumination (150 lx of bright white light) and after 1 h of dark adaptation (0 lx).
FIG. 8.
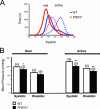
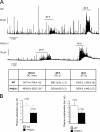
Aortic blood pressures in conscious, adult PHD3 −/− mice, as measured by radiotelemetry. (A) Pooled frequency histogram of systolic blood pressure over the 3-day recording period (using 1 mm Hg bins) from four (wild-type) or five (PHD3 −/−) resting (solid lines) and active (dotted lines) mice. (B) Average blood pressure recordings over a 3-day recording period. Decreased systolic and diastolic blood pressures were observed in PHD3 −/− mice. These differences were enhanced when the mice were active.
FIG. 9.
Catecholamine secretion in PHD3 −/− mice. (A) Representative amperometric recordings of basal catecholamine release, as well as responsiveness to induction by 20 mM and 40 mM potassium (20K and 40K), of adrenal slices isolated from adult mice. The table shows the secretion rate (in fC/min) under basal conditions, as well as with 20 mM and 40 mM potassium (20 and 40 K). (B) Circulating catecholamines (adrenaline and noradrenaline) from anesthetized, adult mice. Both adrenal slice and circulating catecholamines are reduced in PHD3 −/− mice.
Similar articles
- Prolyl-4-hydroxylase domain 3 (PHD3) is a critical terminator for cell survival of macrophages under stress conditions.
Swain L, Wottawa M, Hillemann A, Beneke A, Odagiri H, Terada K, Endo M, Oike Y, Farhat K, Katschinski DM. Swain L, et al. J Leukoc Biol. 2014 Sep;96(3):365-75. doi: 10.1189/jlb.2HI1013-533R. Epub 2014 Mar 13. J Leukoc Biol. 2014. PMID: 24626957 Free PMC article. - Expression of prolyl hydroxylases (PHDs) is selectively controlled by HIF-1 and HIF-2 proteins in nucleus pulposus cells of the intervertebral disc: distinct roles of PHD2 and PHD3 proteins in controlling HIF-1α activity in hypoxia.
Fujita N, Markova D, Anderson DG, Chiba K, Toyama Y, Shapiro IM, Risbud MV. Fujita N, et al. J Biol Chem. 2012 May 11;287(20):16975-86. doi: 10.1074/jbc.M111.334466. Epub 2012 Mar 26. J Biol Chem. 2012. PMID: 22451659 Free PMC article. - Depletion of PHD3 protects heart from ischemia/reperfusion injury by inhibiting cardiomyocyte apoptosis.
Xie L, Pi X, Wang Z, He J, Willis MS, Patterson C. Xie L, et al. J Mol Cell Cardiol. 2015 Mar;80:156-65. doi: 10.1016/j.yjmcc.2015.01.007. Epub 2015 Jan 26. J Mol Cell Cardiol. 2015. PMID: 25633836 Free PMC article. - The regulation, localization, and functions of oxygen-sensing prolyl hydroxylase PHD3.
Jaakkola PM, Rantanen K. Jaakkola PM, et al. Biol Chem. 2013 Apr;394(4):449-57. doi: 10.1515/hsz-2012-0330. Biol Chem. 2013. PMID: 23380539 Review. - Regulation of gene expression by the hypoxia-inducible factors.
Fedele AO, Whitelaw ML, Peet DJ. Fedele AO, et al. Mol Interv. 2002 Jul;2(4):229-43. doi: 10.1124/mi.2.4.229. Mol Interv. 2002. PMID: 14993394 Review.
Cited by
- Deficiency of the oxygen sensor PHD1 augments liver regeneration after partial hepatectomy.
Mollenhauer M, Kiss J, Dudda J, Kirchberg J, Rahbari N, Radhakrishnan P, Niemietz T, Rausch V, Weitz J, Schneider M. Mollenhauer M, et al. Langenbecks Arch Surg. 2012 Dec;397(8):1313-22. doi: 10.1007/s00423-012-0998-5. Epub 2012 Sep 11. Langenbecks Arch Surg. 2012. PMID: 22961008 - New cancer targets emerging from studies of the Von Hippel-Lindau tumor suppressor protein.
Kaelin WG Jr. Kaelin WG Jr. Ann N Y Acad Sci. 2010 Oct;1210:1-7. doi: 10.1111/j.1749-6632.2010.05781.x. Ann N Y Acad Sci. 2010. PMID: 20973793 Free PMC article. - Hypoxia Pathway Proteins in Normal and Malignant Hematopoiesis.
Wielockx B, Grinenko T, Mirtschink P, Chavakis T. Wielockx B, et al. Cells. 2019 Feb 13;8(2):155. doi: 10.3390/cells8020155. Cells. 2019. PMID: 30781787 Free PMC article. Review. - Developmental role of PHD2 in the pathogenesis of pseudohypoxic pheochromocytoma.
Eckardt L, Prange-Barczynska M, Hodson EJ, Fielding JW, Cheng X, Lima JDCC, Kurlekar S, Douglas G, Ratcliffe PJ, Bishop T. Eckardt L, et al. Endocr Relat Cancer. 2021 Oct 18;28(12):757-772. doi: 10.1530/ERC-21-0211. Endocr Relat Cancer. 2021. PMID: 34658364 Free PMC article. - Prolyl-4-hydroxylase 3 (PHD3) expression is downregulated during epithelial-to-mesenchymal transition.
Place TL, Nauseef JT, Peterson MK, Henry MD, Mezhir JJ, Domann FE. Place TL, et al. PLoS One. 2013 Dec 18;8(12):e83021. doi: 10.1371/journal.pone.0083021. eCollection 2013. PLoS One. 2013. PMID: 24367580 Free PMC article.
References
- Appelhoff, R. J., Y. M. Tian, R. R. Raval, H. Turley, A. L. Harris, C. W. Pugh, P. J. Ratcliffe, and J. M. Gleadle. 2004. Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J. Biol. Chem. 27938458-38465. - PubMed
- Aragones, J., M. Schneider, K. Van Geyte, P. Fraisl, T. Dresselaers, M. Mazzone, R. Dirkx, S. Zacchigna, H. Lemieux, N. H. Jeoung, D. Lambrechts, T. Bishop, P. Lafuste, A. Diez-Juan, S. K. Harten, P. Van Noten, K. De Bock, C. Willam, M. Tjwa, A. Grosfeld, R. Navet, L. Moons, T. Vandendriessche, C. Deroose, B. Wijeyekoon, J. Nuyts, B. Jordan, R. Silasi-Mansat, F. Lupu, M. Dewerchin, C. Pugh, P. Salmon, L. Mortelmans, B. Gallez, F. Gorus, J. Buyse, F. Sluse, R. A. Harris, E. Gnaiger, P. Hespel, P. Van Hecke, F. Schuit, P. Van Veldhoven, P. Ratcliffe, M. Baes, P. Maxwell, and P. Carmeliet. 2008. Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nat. Genet. 40170-180. - PubMed
- Barker, D. J. 2002. Fetal programming of coronary heart disease. Trends Endocrinol. Metab. 13364-368. - PubMed
- Barker, D. J., S. P. Bagby, and M. A. Hanson. 2006. Mechanisms of disease: in utero programming in the pathogenesis of hypertension. Nat. Clin. Pract. Nephrol. 2700-707. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- WT_/Wellcome Trust/United Kingdom
- 071251/WT_/Wellcome Trust/United Kingdom
- G0200482/MRC_/Medical Research Council/United Kingdom
- BHF_/British Heart Foundation/United Kingdom
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases