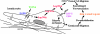Transforming growth factor alpha (TGFalpha)-stimulated secretion of HSP90alpha: using the receptor LRP-1/CD91 to promote human skin cell migration against a TGFbeta-rich environment during wound healing - PubMed (original) (raw)
. 2008 May;28(10):3344-58.
doi: 10.1128/MCB.01287-07. Epub 2008 Mar 10.
Jianhua Fan, Mark Fedesco, Shengxi Guan, Yong Li, Balaji Bandyopadhyay, Alexandra M Bright, Dalia Yerushalmi, Mengmeng Liang, Mei Chen, Yuan-Ping Han, David T Woodley, Wei Li
Affiliations
- PMID: 18332123
- PMCID: PMC2423165
- DOI: 10.1128/MCB.01287-07
Transforming growth factor alpha (TGFalpha)-stimulated secretion of HSP90alpha: using the receptor LRP-1/CD91 to promote human skin cell migration against a TGFbeta-rich environment during wound healing
Chieh-Fang Cheng et al. Mol Cell Biol. 2008 May.
Erratum in
- Mol Cell Biol. 2012 Jan;32(1):240
Abstract
Jump-starting and subsequently maintaining epidermal and dermal cell migration are essential processes for skin wound healing. These events are often disrupted in nonhealing wounds, causing patient morbidity and even fatality. Currently available treatments are unsatisfactory. To identify novel wound-healing targets, we investigated secreted molecules from transforming growth factor alpha (TGFalpha)-stimulated human keratinoytes, which contained strong motogenic, but not mitogenic, activity. Protein purification allowed us to identify the heat shock protein 90alpha (hsp90alpha) as the factor fully responsible for the motogenic activity in keratinocyte secretion. TGFalpha causes rapid membrane translocation and subsequent secretion of hsp90alpha via the unconventional exosome pathway in the cells. Secreted hsp90alpha promotes both epidermal and dermal cell migration through the surface receptor LRP-1 (LDL receptor-related protein 1)/CD91. The promotility activity resides in the middle domain plus the charged sequence of hsp90alpha but is independent of the ATPase activity. Neutralizing the extracellular function of hsp90alpha blocks TGFalpha-induced keratinicyte migration. Most intriguingly, unlike the effects of canonical growth factors, the hsp90alpha signaling overrides the inhibition of TGFbeta, an abundant inhibitor of dermal cell migration in skin wounds. This finding provides a long-sought answer to the question of how dermal cells migrate into the wound environment to build new connective tissues and blood vessels. Thus, secreted hsp90alpha is potentially a new agent for wound healing.
Figures
FIG. 1.
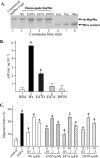
Secretion of TGFα-treated HKCs promotes skin cell migration but not proliferation. (A) Outline for preparing serum-free HKC-CM and control medium (Con). (B) Comparison of HKC-CM with TGFα in stimulation of HKC migration by use of a colloidal gold migration assay (see Materials and Methods) (n = 4; P < 0.05). A migration track representing the average size under each set of conditions is marked with an open circle. (C) Comparison of HKC-CM with TGFα with respect to HKC migration in the “scratch” assay. AG, average gap (see Materials and Methods) (n = 3, P < 0.05). (D) Unlike serum growth factors, HKC-CM did not cause DNA synthesis in the major human skin cell types represented by HKCs, DFs, and HDMECs (n = 4). Bars marked with an asterisk represent statistically significant results compared to those seen with serum-free controls (P < 0.01). (E) Comparison of HKC-CM to serum growth factors with respect to migration of HKCs, DFs, and HDMECs by use of a colloidal gold migration assay. Data represent migration index (MI) results from the experiments (n = 3). Bars with an asterisk represent statistically significant results compared to those seen with serum-free controls (P < 0.05).
FIG. 2.
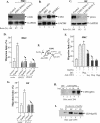
Identification of hsp90α from HKC-CM. (A) A detailed description of the procedure for protein purification and a data summary are provided in Table S2 in the supplemental material. HKC-CM (20×; 50 μl) was analyzed by SDS-PAGE and silver staining (left column). Migration assays were used to identify the “positive” fraction(s) of purification that contained the peak of promotility activity. Pooled and concentrated materials of fractions 17 and 18 from the final Superdex 200 chromatography procedure were resolved in SDS gel and silver stained (right column). The four identifiable bands were excised and subjected to mass spectrometry analyses, and their identities are shown (arrows). FN, fibronectin. (B) Recombinant proteins of human hsp90α, AMF, and 14-3-3σ were individually tested for stimulation of HKC migration, and the results were compared to those seen with stimulation of TGFα. The results of the colloidal gold migration assays (n = 3) are shown as migration index values. Values represented by bars with asterisks were statistically significant in comparisons with control values (P < 0.05). (C) Samples with known amounts of recombinant hsp90α, as indicated, were loaded side by side with increasing volumes of 10× HKC-CM on an SDS gel and subjected to immunoblotting analysis with an anti-hsp90α antibody. A standard curve was established based on densitometry scanning of the control bands and was used to estimate the amount of hsp90α in HKC-CM (n = 3). (D) Neutralizing antibodies against human hsp90α (bars 6 to 10), AMF (bar 4), 14-3-3σ (bar 5), or control IgG (bar 3) were used to “deplete” each of the corresponding antigens in HKC-CM by immunoprecipitation. The treated HKC-CMs were tested for their stimulation of HKC migration in colloidal gold migration assays. Only the migration index values obtained in the experiments (n = 4) are shown. Bars with asterisks represent statistical decreases compared to the results obtained with the positive controls (P < 0.01).
FIG. 3.
Hsp90α is secreted selectively by HKCs and has no mitogenic effect. (A) Equal volumes of serum-free CM from four major types of primary human skin cells (HKCs, DFs, HDMECs [End.], and MCs) were analyzed by Western immunoblotting with anti-hsp90α antibodies. Commercial human recombinant (Recomb.) hsp90α protein was included as a positive control. (B) Serum-starved HKCs, DFs, and HDMECs were left untreated or were treated with indicated stimuli in [3H]thymidine incorporation assays. The data are presented as increases based on triplicate experiments per set of conditions. Arrows point to the effect of hsp90α (bars 7 to 9) in comparison to the expected mitogenic effect of GF stimulation (bars 4 to 6) (n = 3). Bars with asterisks represent statistically significant differences in comparison to the results obtained with serum-free controls (P < 0.01). (C) Comparison of the total cellular levels of hsp90α (a and b) versus hsp90β (c and d) among epidermal HKCs, DFs, and HDMECs in Western blot analyses with anti-hsp90α antibodies. These experiments were repeated four times, and similar observations were made each time. (D) Normal human skin sections were subjected to indirect immunofluorescence staining with antibodies against hsp90α (b) or hsp90β (d) or corresponding IgG controls (a and c). The images show the skin tissue distribution of hsp90α (b) and hsp90β (d). Dotted lines refer to the basement membrane zone of skin. Derm, dermis; Epi, epidermis.
FIG. 4.
TGFα stimulates membrane translocation and secretion of endogenous hsp90α selectively in HKCs. (A) HKCs, cultured on collagen-coated coverslips and starved of serum overnight, were either left untreated or treated with an optimal concentration of TGFα (20 ng/ml) for the indicated periods of time (in minutes) (also see detailed kinetics in Fig. S3 in the supplemental material). In the same experiments, PDGF-BB-treated DFs were included as a cell type specificity control. Both cell types were fixed and immunostained with a monoclonal antibody specifically against human hsp90α. The results were visualized by use of fluorescence-conjugated secondary antibody. A total of 80 to 120 cells per set of conditions were randomly selected and analyzed. For each set of conditions, a representative image and the percentage of the cells represented in the image compared to the total number of the cells examined are shown. (B) HKCs and DFs were simultaneously cultured, serum starved, and treated with TGFα. As shown, TGFα stimulates hsp90α membrane translocation selectively in HKCs but not in DFs. (C) Serum-starved HKCs were left untreated or were treated with 20 ng/ml TGFα for 4 h. Both total lysates and equal volumes of serum-free conditioned media (concentrated from 4 ml to 50 μl prior to gel analysis) were subjected to Western blotting with an anti-hsp90α antibody. TGFα-induced decreases in the level of intracellular hspα versus the untreated cell level were averaged based on results of four independent experiments (n = 4; P < 0.05).
FIG. 5.
The exosome pathway mediates TGFα-induced membrane translocation and secretion of hsp90α in HKCs. (A) HKCs were either left untreated or treated with TGFα (20 ng/ml) in the absence or presence of the indicated concentrations of DMA or BFA for 15 min. The experiments and data quantitation were carried out as described for Fig. 4A. (B) Conditioned media (concentrated from 4 ml to 50 μl prior to gel analysis) of the cells, either left untreated or treated with 20 ng/ml TGFα in the absence or presence of DMA or BFA for 4 h, were subjected to Western blotting with anti-hsp90α antibodies. Equal loading of the samples was controlled at three levels: (i) equal numbers of the cells were maintained in different dishes of culture; (ii) equal volumes of the media were added to the dishes; and (iii) further calibration of the volumes of loading samples against the cell numbers at the end of the incubation was performed.
FIG. 6.
Extracellular hsp90α promotes cell migration independently of ATP binding or ATPase activity. (A) Purified wt and mutant human recombinant hsp90α (hrHsp90α) proteins (wt, E47D, E47A, and D93N) were subjected to an SDS-PAGE analysis together with a commercial hsp90α (1 μg) and a series of known amounts (1 to 10 μg) of BSA. The proteins were visualized by Coomassie blue staining. (B) The recombinant proteins were subjected to in vitro ATPase assays (see Materials and Methods), using BSA as the baseline control. (C) The wt, E47D, E47A, and D93N mutant hsp90α proteins showed similar dose-dependent promotility effects on HKCs. Only the migration indices are shown. Asterisks indicate values statistically significantly different compared with the control (n = 5; P < 0.05).
FIG. 7.
Hsp90α promotes HKC migration mainly through its middle domain. In similarity to the procedures described for Fig. 6, purified wt and the indicated fragments of human hsp90α proteins were verified by SDS-PAGE and Coomassie blue staining (A) and subjected to colloidal gold migration assays (0.1 μM each) in comparison to control serum-free medium results (B). Only the migration indices (MI) are shown (n = 3).
FIG. 8.
CD91 receptor mediates hsp90α signaling to promote cell migration. (A) Lysates of HKCs infected with lentivirus carrying either a control siRNA (LacZ-siRNA [Control]) or two siRNAs against CD91 (CD91-RNAi-1 and CD91-RNAi-2) were analyzed by Western blotting with an anti-CD91 antibody. (B) CD91 expression in HKCs, DFs, and HDMECs, in comparison to dendritic cell results, was determined by Western blot analyses. (C) Lysates of DFs infected with lentivirus carrying either a control siRNA (LacZ-siRNA [Control]) or the siRNAs against CD91 were analyzed by Western blotting with an anti-CD91 antibody. (D) The effects of down-regulation of CD91 by two distinct siRNAs on HKC migration in response to hsp90α (10 μg/ml) were analyzed in colloidal gold migration assays. Bars with asterisks represent statistically significant results in comparison with serum-free control results (n = 4; P < 0.03). (E) Schematic presentation of how neutralizing anti-CD91 antibodies block the cell surface CD91 to prevent hsp90α binding. (F) Effect of anti-CD91 blocking antibody on HKC migration in response to hsp90α. HKCs on collagen in colloidal gold migration assays were preincubated with increasing concentrations of an anti-CD91 neutralizing antibody (which was maintained as a continued presence throughout the assays) for 30 min or with control IgG prior to addition of hsp90α. (G) The effects of RNAi down-regulation of CD91 on DF migration in response to hsp90α (10 μg/ml) were analyzed in colloidal gold migration assays. Bars with asterisks represent statistically significant results in comparison with serum-free control results (n = 3; P < 0.05). (H) Lysates of HKCs were incubated with the indicated amounts of GST-hsp90α fusion proteins or with GST alone on beads. Bead-bound proteins were dissociated and analyzed by Western blotting with an anti-CD91 antibody. (I) Lysates of HKCs were incubated with 20 μg each of three His-tagged domains of hsp90α on Ni beads. The bound proteins were analyzed by Western blotting with an anti-CD91 antibody (n = 3; P < 0.05). Con., control; FL, full length.
FIG. 9.
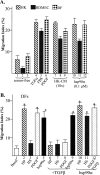
The extracellular function of hsp90α is essential for HK migration. (A and B) HKCs were subjected to colloidal gold migration assays in response to TGFα (20 ng/ml) (A) or 10% HS (B) in the absence or presence of increasing amounts of functionally neutralizing anti-hsp90α antibodies (far right column). The migration index (MI) values were determined on the basis of three independent experiments (n = 3; P, 0.03 to 0.01). The average-sized migration tracks are marked with arrows (pointing to the cell). (C) Included as a control, migration of DFs in response to PDGF-BB was not inhibited by the same antibodies (panels m-q), n = 4, P < 0.05.
FIG. 10.
Extracellular hsp90α bypasses the inhibitory effect of TGFβ on human dermal cell migration. Primary human DFs were starved of serum overnight and subjected to colloidal gold migration assays (under either untreated or treated conditions) with the various reagents, including HP (3%), HS (3%), PDGF-BB (15 ng/ml), and hsp90α (0.1 μM), in the absence or presence of TGFβ3 (0.4 ng/ml), the critical inhibitor of dermal cell migration present in human serum (2). Only the migration index values are shown (n = 3; P < 0.05). Bars with asterisks represent statistically significant results in comparison with serum-free control results.
FIG. 11.
A schematic summary: the TGFα → hsp90α secretion → skin cell migration → wound healing model. Following skin injury, paracrine- or autocrine-released TGFα stimulates membrane translocation and secretion of the preexisting hsp90α proteins in HKCs. Secreted hsp90α jump-starts HKC migration, a critical event of the reepithelialization process, by binding to the LRP-1/CD91 receptor on the cell surface. After the extracellular hsp90α also defuses into and reaches a certain concentration in the wound bed, it induces migration of DFs and HDMECs from the cut edge into the wound bed even under “hazard” conditions (i.e., no adequate concentrations of ATP and the presence of pontent motility inhibitors, such as TGFβ). Thus, extracellular hsp90α may be a useful target for skin wound healing. ECM, extracellular matrix.
Similar articles
- Extracellular heat shock protein-90alpha: linking hypoxia to skin cell motility and wound healing.
Li W, Li Y, Guan S, Fan J, Cheng CF, Bright AM, Chinn C, Chen M, Woodley DT. Li W, et al. EMBO J. 2007 Mar 7;26(5):1221-33. doi: 10.1038/sj.emboj.7601579. Epub 2007 Feb 15. EMBO J. 2007. PMID: 17304217 Free PMC article. - Participation of the lipoprotein receptor LRP1 in hypoxia-HSP90alpha autocrine signaling to promote keratinocyte migration.
Woodley DT, Fan J, Cheng CF, Li Y, Chen M, Bu G, Li W. Woodley DT, et al. J Cell Sci. 2009 May 15;122(Pt 10):1495-8. doi: 10.1242/jcs.047894. Epub 2009 Apr 21. J Cell Sci. 2009. PMID: 19383717 Free PMC article. - Role for heat shock protein 90α in the proliferation and migration of HaCaT cells and in the deep second-degree burn wound healing in mice.
Zhang Y, Bai X, Wang Y, Li N, Li X, Han F, Su L, Hu D. Zhang Y, et al. PLoS One. 2014 Aug 11;9(8):e103723. doi: 10.1371/journal.pone.0103723. eCollection 2014. PLoS One. 2014. PMID: 25111496 Free PMC article. - Keratinocyte Migration and a Hypothetical New Role for Extracellular Heat Shock Protein 90 Alpha in Orchestrating Skin Wound Healing.
Woodley DT, Wysong A, DeClerck B, Chen M, Li W. Woodley DT, et al. Adv Wound Care (New Rochelle). 2015 Apr 1;4(4):203-212. doi: 10.1089/wound.2014.0566. Adv Wound Care (New Rochelle). 2015. PMID: 25945283 Free PMC article. Review. - Keratinocyte-Secreted Heat Shock Protein-90alpha: Leading Wound Reepithelialization and Closure.
Bhatia A, O'Brien K, Chen M, Woodley DT, Li W. Bhatia A, et al. Adv Wound Care (New Rochelle). 2016 Apr 1;5(4):176-184. doi: 10.1089/wound.2014.0620. Adv Wound Care (New Rochelle). 2016. PMID: 27076995 Free PMC article. Review.
Cited by
- Therapeutic effect of mesenchymal stem cells on histopathological, immunohistochemical, and molecular analysis in second-grade burn model.
Abdel-Gawad DRI, Moselhy WA, Ahmed RR, Al-Muzafar HM, Amin KA, Amin MM, El-Nahass ES, Abdou KAH. Abdel-Gawad DRI, et al. Stem Cell Res Ther. 2021 May 29;12(1):308. doi: 10.1186/s13287-021-02365-y. Stem Cell Res Ther. 2021. PMID: 34051875 Free PMC article. - Plasma Hsp90 Level as a Marker of Early Acute Lymphoblastic Leukemia Engraftment and Progression in Mice.
Milani M, Laranjeira AB, de Vasconcellos JF, Brandalise SR, Nowill AE, Yunes JA. Milani M, et al. PLoS One. 2015 Jun 11;10(6):e0129298. doi: 10.1371/journal.pone.0129298. eCollection 2015. PLoS One. 2015. PMID: 26068922 Free PMC article. - Targeting HSP90 as a Novel Therapy for Cancer: Mechanistic Insights and Translational Relevance.
Zhang J, Li H, Liu Y, Zhao K, Wei S, Sugarman ET, Liu L, Zhang G. Zhang J, et al. Cells. 2022 Sep 6;11(18):2778. doi: 10.3390/cells11182778. Cells. 2022. PMID: 36139353 Free PMC article. Review. - Endothelial-mesenchymal transition harnesses HSP90α-secreting M2-macrophages to exacerbate pancreatic ductal adenocarcinoma.
Fan CS, Chen LL, Hsu TA, Chen CC, Chua KV, Li CP, Huang TS. Fan CS, et al. J Hematol Oncol. 2019 Dec 17;12(1):138. doi: 10.1186/s13045-019-0826-2. J Hematol Oncol. 2019. PMID: 31847880 Free PMC article. - Effect of the secretory small GTPase Rab27B on breast cancer growth, invasion, and metastasis.
Hendrix A, Maynard D, Pauwels P, Braems G, Denys H, Van den Broecke R, Lambert J, Van Belle S, Cocquyt V, Gespach C, Bracke M, Seabra MC, Gahl WA, De Wever O, Westbroek W. Hendrix A, et al. J Natl Cancer Inst. 2010 Jun 16;102(12):866-80. doi: 10.1093/jnci/djq153. Epub 2010 May 18. J Natl Cancer Inst. 2010. PMID: 20484105 Free PMC article.
References
- Ali, J. A., A. Jackson, P. Howells, and A. J. Maxwell. 1993. The 43-kilodalton N-terminal fragment of the DNA gyrase B protein hydrolyzes ATP and binds coumarin drugs. Biochemistry 322717-2724. - PubMed
- Basu, S., R. J. Binder, R. Suto, K. M. Anderson, and P. K. Srivastava. 2000. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-κB pathway. Int. Immunol. 121539-1546. - PubMed
- Basu, S., R. J. Binder, T. Ramalingam, and P. K. Srivastava. 2001. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity 14303-313. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AR046538/AR/NIAMS NIH HHS/United States
- R01 AR066193/AR/NIAMS NIH HHS/United States
- AR46538/AR/NIAMS NIH HHS/United States
- GM/AR066193-01/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous