Bardet-Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia - PubMed (original) (raw)
Bardet-Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia
Nicolas F Berbari et al. Proc Natl Acad Sci U S A. 2008.
Abstract
Primary cilia are ubiquitous cellular appendages that provide important yet not well understood sensory and signaling functions. Ciliary dysfunction underlies numerous human genetic disorders. However, the precise defects in cilia function and the basis of disease pathophysiology remain unclear. Here, we report that the proteins disrupted in the human ciliary disorder Bardet-Biedl syndrome (BBS) are required for the localization of G protein-coupled receptors to primary cilia on central neurons. We demonstrate a lack of ciliary localization of somatostatin receptor type 3 (Sstr3) and melanin-concentrating hormone receptor 1 (Mchr1) in neurons from mice lacking the Bbs2 or Bbs4 gene. Because Mchr1 is involved in the regulation of feeding behavior and BBS is associated with hyperphagia-induced obesity, our results suggest that altered signaling caused by mislocalization of ciliary signaling proteins underlies the BBS phenotypes. Our results also provide a potential molecular mechanism to link cilia defects with obesity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
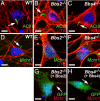
BBS mice possess neuronal primary cilia in the brain but lack Sstr3-positive cilia. (A–C) Representative images of the CA3 region of the hippocampus in adult WT (A), _Bbs2_−/− (B), and _Bbs4_−/− (C) mice (n = 9) showing labeling for ACIII (red). Nuclei are stained with DRAQ5 (blue). The appearance and distribution of ACIII-positive cilia are similar among all genotypes. (D–F) The identical fields showing labeling for Sstr3 (green) reveals Sstr3-positive cilia in the WT (D) section but a complete lack of Sstr3-positive cilia in the _Bbs2_−/− (E) or _Bbs4_−/− (F) sections. (G–I) The merged images showing colocalization of ACIII and Sstr3 to cilia in the WT (G) section and no Sstr3 labeling of cilia in the _Bbs2_−/− (H) or _Bbs4_−/− (I) sections. (Scale bars, 10 μm.)
Fig. 2.
Sstr3 ciliary localization can be restored in vitro. (A–C) Coimmunolabeling of day 7 hippocampal neurons from WT (A), _Bbs2_−/− (B), and _Bbs4_−/− (C) mice with antibodies to ACIII (green) and βTIII (red) shows the presence of cilia (arrows) in all three genotypes. (D–F) Coimmunolabeling of day 7 hippocampal neurons from WT (D), _Bbs2_−/− (E), and _Bbs4_−/− (F) mice with antibodies to Sstr3 (green) and βTIII (red) shows the presence of cilia (arrow) only on the WT neuron. Note the apparent punctate Sstr3 labeling in the _Bbs2_−/− (E) and _Bbs4_−/− (F) neurons. (G and H) Immunolabeling of day 7 hippocampal neurons from _Bbs2_−/− (G) and _Bbs4_−/− (H) mice for Sstr3 (red) 2 days posttransfection with an expression vector encoding Bbs2 and Bbs4, respectively, and a vector expressing enhanced green fluorescence protein (GFP; green) as a transfection marker. Note that heterologous expression of BBS proteins restores Sstr3 ciliary labeling (arrows). Nuclei are stained with DRAQ5 (blue). (Scale bars, 5 μm.)
Fig. 3.
BBS mice lack Mchr1 ciliary labeling in the brain. (A–C) Representative images of the nucleus accumbens in adult WT (A), _Bbs2_−/− (B), and _Bbs4_−/− (C) mice (n = 9) showing labeling for ACIII (red). The appearance and distribution of ACIII-positive cilia are similar among all genotypes. (D–F) The identical fields showing labeling for Mchr1 (green) reveals Mchr1-positive cilia in the WT (D) section but a complete lack of Mchr1-positive cilia in the _Bbs2_−/− (E) or _Bbs4_−/− (F) sections. Note the presence of increased punctate labeling in the _Bbs2_−/− (E) and _Bbs4_−/− (F) sections. (G–I) The merged images showing colocalization of ACIII and Mchr1 to cilia in the WT (G) section and no Mchr1 labeling of cilia in the _Bbs2_−/− (H) or _Bbs4_−/− (I) sections. Nuclei are stained with DRAQ5 (blue). (Scale bars, 10 μm.)
Fig. 4.
Mchr1 ciliary localization can be restored in vitro. (A–C) Coimmunolabeling of day 7 nucleus accumbens/olfactory tubercle-enriched neurons from WT (A), _Bbs2_−/− (B), and _Bbs4_−/− (C) mice with antibodies to ACIII (green) and βTIII (red). Cilia (arrows) are present in all three genotypes. (D–F) Coimmunolabeling of day 7 nucleus accumbens/olfactory tubercle-enriched neurons from WT (D), _Bbs2_−/− (E), and _Bbs4_−/− (F) mice with antibodies to Mchr1 (green) and βTIII (red) shows the presence of cilia (arrow) only on the WT neuron. Note the punctate staining in E and F. (G and H) Immunolabeling of day 7 hypothalamic neurons from _Bbs2_−/− (G) and _Bbs4_−/− (H) mice for Mchr1 (red) 2 days posttransfection with an expression vector encoding Bbs2 and Bbs4, respectively, and a vector expressing enhanced green fluorescence protein (GFP; green) as a transfection marker. Note that heterologous expression of BBS proteins restores Mchr1 ciliary labeling (arrows). Nuclei are stained with DRAQ5 (blue). (Scale bars, 5 μm.)
Similar articles
- Dopamine receptor 1 localizes to neuronal cilia in a dynamic process that requires the Bardet-Biedl syndrome proteins.
Domire JS, Green JA, Lee KG, Johnson AD, Askwith CC, Mykytyn K. Domire JS, et al. Cell Mol Life Sci. 2011 Sep;68(17):2951-60. doi: 10.1007/s00018-010-0603-4. Epub 2010 Dec 9. Cell Mol Life Sci. 2011. PMID: 21152952 Free PMC article. - Bardet-Biedl syndrome type 4 (BBS4)-null mice implicate Bbs4 in flagella formation but not global cilia assembly.
Mykytyn K, Mullins RF, Andrews M, Chiang AP, Swiderski RE, Yang B, Braun T, Casavant T, Stone EM, Sheffield VC. Mykytyn K, et al. Proc Natl Acad Sci U S A. 2004 Jun 8;101(23):8664-9. doi: 10.1073/pnas.0402354101. Epub 2004 Jun 1. Proc Natl Acad Sci U S A. 2004. PMID: 15173597 Free PMC article. - Ectopic expression of human BBS4 can rescue Bardet-Biedl syndrome phenotypes in Bbs4 null mice.
Chamling X, Seo S, Bugge K, Searby C, Guo DF, Drack AV, Rahmouni K, Sheffield VC. Chamling X, et al. PLoS One. 2013;8(3):e59101. doi: 10.1371/journal.pone.0059101. Epub 2013 Mar 15. PLoS One. 2013. PMID: 23554981 Free PMC article. - Bardet-Biedl syndrome: Is it only cilia dysfunction?
Novas R, Cardenas-Rodriguez M, Irigoín F, Badano JL. Novas R, et al. FEBS Lett. 2015 Nov 14;589(22):3479-91. doi: 10.1016/j.febslet.2015.07.031. Epub 2015 Jul 29. FEBS Lett. 2015. PMID: 26231314 Review. - Establishing a connection between cilia and Bardet-Biedl Syndrome.
Mykytyn K, Sheffield VC. Mykytyn K, et al. Trends Mol Med. 2004 Mar;10(3):106-9. doi: 10.1016/j.molmed.2004.01.003. Trends Mol Med. 2004. PMID: 15106604 Review.
Cited by
- End stage renal disease, differential diagnosis, a rare genetic disorder: bardet-biedl syndrome: case report and review.
Sowjanya B, Sreenivasulu U, Naidu JN, Sivaranjani N. Sowjanya B, et al. Indian J Clin Biochem. 2011 Apr;26(2):214-6. doi: 10.1007/s12291-011-0116-4. Epub 2011 Feb 4. Indian J Clin Biochem. 2011. PMID: 22468053 Free PMC article. - Cilia in the Striatum Mediate Timing-Dependent Functions.
Alhassen W, Alhassen S, Chen J, Monfared RV, Alachkar A. Alhassen W, et al. Mol Neurobiol. 2023 Feb;60(2):545-565. doi: 10.1007/s12035-022-03095-9. Epub 2022 Nov 2. Mol Neurobiol. 2023. PMID: 36322337 Free PMC article. - Trafficking in and to the primary cilium.
Hsiao YC, Tuz K, Ferland RJ. Hsiao YC, et al. Cilia. 2012 Apr 25;1(1):4. doi: 10.1186/2046-2530-1-4. Cilia. 2012. PMID: 23351793 Free PMC article. - Diffusion rather than intraflagellar transport likely provides most of the tubulin required for axonemal assembly in Chlamydomonas.
Craft Van De Weghe J, Harris JA, Kubo T, Witman GB, Lechtreck KF. Craft Van De Weghe J, et al. J Cell Sci. 2020 Sep 11;133(17):jcs249805. doi: 10.1242/jcs.249805. J Cell Sci. 2020. PMID: 32801124 Free PMC article. - Exploring Key Challenges of Understanding the Pathogenesis of Kidney Disease in Bardet-Biedl Syndrome.
Marchese E, Ruoppolo M, Perna A, Capasso G, Zacchia M. Marchese E, et al. Kidney Int Rep. 2020 Jun 29;5(9):1403-1415. doi: 10.1016/j.ekir.2020.06.017. eCollection 2020 Sep. Kidney Int Rep. 2020. PMID: 32954066 Free PMC article. Review.
References
- Wheatley DN, Wang AM, Strugnell GE. Expression of primary cilia in mammalian cells. Cell Biol Int. 1996;20:73–81. - PubMed
- Pan J, Wang Q, Snell WJ. Cilium-generated signaling and cilia-related disorders. Lab Invest. 2005;85:452–463. - PubMed
- Singla V, Reiter JF. The primary cilium as the cell's antenna: Signaling at a sensory organelle. Science. 2006;313:629–633. - PubMed
- Marshall WF, Nonaka S. Cilia: Tuning in to the cell's antenna. Curr Biol. 2006;16:R604–R614. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases