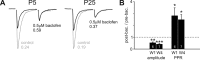Functional maturation of the first synapse in olfaction: development and adult neurogenesis - PubMed (original) (raw)
Comparative Study
Functional maturation of the first synapse in olfaction: development and adult neurogenesis
Matthew S Grubb et al. J Neurosci. 2008.
Abstract
The first synapse in olfaction undergoes considerable anatomical plasticity in both early postnatal development and adult neurogenesis, yet we know very little concerning its functional maturation at these times. Here, we used whole-cell recordings in olfactory bulb slices to describe olfactory nerve inputs to developing postnatal neurons and to maturing adult-born cells labeled with a GFP-encoding lentivirus. In both postnatal development and adult neurogenesis, the maturation of olfactory nerve synapses involved an increase in the relative contribution of AMPA over NMDA receptors, and a decrease in the contribution of NMDA receptors containing the NR2B subunit. These postsynaptic transformations, however, were not mirrored by presynaptic changes: in all cell groups, paired-pulse depression remained constant as olfactory nerve synapses matured. Although maturing cells may therefore offer, transiently, a functionally distinct connection for inputs from the nose, presynaptic function at the first olfactory connection remains remarkably constant in the face of considerable anatomical plasticity.
Figures
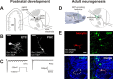
Figure 1.
Recording evoked olfactory nerve inputs in three types of maturing OB glomerular layer neurons. A, Schematic of our experimental approach for developing postnatal juxtaglomerular cells (JGCs). Acute horizontal slices were cut from mouse olfactory bulb, and a monopolar stimulating electrode was placed in the olfactory nerve layer. Evoked inputs from OSN axons were then recorded via a patch pipette placed on a juxtaglomerular cell. B, Biocytin fills illustrating the two types of developing juxtaglomerular cell recorded. ETCs were large, with correspondingly low _R_m and high _C_m. PGCs were much smaller, with high _R_m and low _C_s. Scale bar, 20 μm. C, Sodium currents recorded in an ETC (left) and a PGC (right) after depolarization to −20 mV. Whereas ETCs displayed multiple currents, PGCs displayed only one. D, Schematic of our experimental approach for adult-born periglomerular cells. Newly born cells migrating toward the OB were labeled in adult mice via stereotaxic injection of a GFP-expressing lentivirus into the RMS. We then patched GFP+ cells in the glomerular layer and recorded evoked olfactory nerve inputs as shown above. LV, Lateral ventricle. E, An example of a recorded adult-born PGC. The biocytin fill (red) showed typical PGC morphology, whereas subsequent immunohistochemical staining for GFP (green) showed that the recorded cell was indeed newly generated. TOTO (blue) labels cell bodies and shows glomerular structure; the recorded cell's glomerulus is outlined in white. Scale bar, 20 μm.
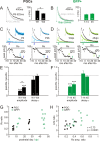
Figure 2.
The AMPA:NMDA ratio at ON synapses increases with postnatal maturation of ETCs and PGCs and with maturation of adult-born GFP+ neurons. A, Calculating an AMPA:NMDA ratio from ON-evoked inputs in a PGC. At −70 mV, in the presence of gabazine to block GABAA receptors, magnesium blockade of NMDA receptors means that the recorded current is AMPA dominated. An estimate of AMPA amplitude (amp1) was therefore taken as the peak of this response. At +40 mV, however, magnesium block is removed from NMDA receptors, revealing a mixed AMPA+NMDA response. Because AMPA responses are fast, as illustrated by the gray trace showing the AMPA-only trace after
l
-APV application (50 μ
m
), an estimate of NMDA amplitude (amp2) could be taken from this combined response at 50 ms after stimulation. The AMPA:NMDA (A:N) ratio was then simply calculated as amp1/amp2. B, Representative traces showing A:N ratios at different stages of maturation in ETCs, PGCs, and adult-generated GFP+ neurons. Bottom traces show responses at −70 mV; top traces show responses at +40 mV. Traces for each cell are normalized by amp1, and the A:N ratio is displayed at the bottom right of each example, showing the clear increase in A:N ratio as maturation proceeds in all three cell types. C, Significant positive correlations between maturation stage and A:N ratio for all three cell types. r and p report results of a nonparametric rank correlation on each dataset. A, Adult. D, Group comparisons show increasing A:N ratios with maturation in all three cell types. In this and in subsequent figures, data plotted are mean ± SEM, and values within bars show sample sizes (n). *p < 0.05; **p < 0.01. W1, Postnatal week 1; W4, postnatal week 4; 7–14, 45, and 90 refer to GFP+ cell groupings based on dpi; GFP− refers to control PGCs in adult tissue.
Figure 3.
ON input NMDA decay kinetics and NR2B subunit contributions in maturing postnatal and adult-born PGCs. A, B, NMDA decay kinetics quicken with the postnatal maturation of PGCs, but do not change significantly with maturation of adult-born GFP+ neurons. Left, Representative NMDA currents recorded at +40 mV and normalized by peak amplitude, from immature (dark) and mature (light) cells. Values show the responses' τ calculated from a single-exponential fit to each curve. Right, Group comparisons showing a significant difference in τ (*p < 0.05) between mature and immature groups for developing PGCs, but not for GFP+ cells. W1, Postnatal week 1; W4, postnatal week 4; 7–14 and 45 refer to GFP+ cell groupings based on dpi. C, D, Partial blockade of NMDA responses with the NR2B subunit-selective drug Ro (0.5 μ
m
) in immature and mature PGCs. Top traces show NMDA responses before (1) and after (2) Ro application, and after complete blockade of the response in APV (50 μ
m
; 3). Significant reduction of NMDA responses was observed with Ro application for both immature and mature neurons, and for both early postnatal and adult-born GFP+ PGCs. Ro appeared, however, to decrease immature NMDA responses a little more for both cell types. Traces for each cell are normalized by initial NMDA response amplitude. Insets show pre-Ro and post-Ro responses normalized by peak amplitude; no consistent change in decay kinetics was observed in any cell group after Ro application. Bottom plots show peak NMDA response amplitude as a function of time after Ro application. E, F, Effects of Ro application on NMDA response amplitude and decay kinetics in immature and mature PGC cell groups. Ro significantly decreased NMDA response amplitude in both immature and mature groups, in both developing postnatal PGCs and adult-born GFP+ cells (***p < 0.001). In developing postnatal PGCs, this decrease was significantly greater in the immature group (*p < 0.05). In adult-born GFP+ PGCs, the same trend was apparent, but the difference was not significant. Ro application did not significantly alter NMDA decay kinetics in any group. G, Population Ro ratio data for both PGC and GFP+ groups. Note the trend toward increased values with maturation for both groups, the higher Ro ratio values in GFP+ cells, and the rapid maturation within the GFP + 7–14 dpi group. H, A strong positive correlation between the reduction in NMDA response amplitude after Ro application (Ro amp. ratio) and AMPA:NMDA ratio measured as shown in supplemental Figure 2_A_ (available at
as supplemental material) (A:N ratio). Cells from all age groups are shown. r and p report the results of a nonparametric rank correlation on all data points; individual correlations for both developing PGCs and adult-born GFP+ cells were also positive and significant.
Figure 4.
No maturation in AMPA decay kinetics in any cell group. A, Representative evoked AMPA responses, recorded at −70 mV, for immature and mature stages in all cell groups. Traces are normalized by peak amplitude; values show the decay constant, τ, resulting from a single-exponential fit. B, No significant correlation of AMPA τ with age or dpi for any cell group. r and p report the results of nonparametric rank correlations. A, Adult. C, No significant group differences in AMPA τ for any cell type. W1, Postnatal week 1; W4, postnatal week 4; 7–14, 45, and 90 refer to GFP+ cell groupings based on dpi.
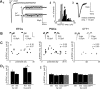
Figure 5.
No increase in ON input quantal size with postnatal maturation of ETCs and PGCs, nor with maturation of adult-born GFP+ neurons. A, Estimating quantal size at ON inputs by replacing external Ca2+ with Sr2+. A1, Sr2+ (2 m
m
) produces asynchronous glutamate release: a single, smooth evoked EPSC recorded in an ETC at P2 is converted into a series of small asynchronous SrEPSCs extended in time. Between 40 and 240 ms after stimulation, shown by the gray box, these events were consistent in amplitude, and were much higher in frequency than background spontaneous events. A2, Histogram of SrEPSC amplitudes recorded in this cell (black). Recording noise amplitudes are shown in white. The arrow points to the median SrEPSC amplitude, taken as an estimate of quantal size for each cell (here 14.3 pA). A3, Average SrEPSC for this example cell. B, Average SrEPSCs for different cell groups at immature and mature time points. Values at bottom show median SrEPSC amplitude for that cell. Calibration: 10 pA, 10 ms. C, No significant correlation of quantal size with maturation in any cell type. r and p report results of nonparametric rank correlations. Each dot represents one cell. D, Group comparisons of quantal size, by cell and by event. D1, In ETCs, when data are combined by cell (taking the median SrEPSC event amplitude per cell), a nonsignificant decrease in ON input quantal size is seen between postnatal week 1 (W1) and postnatal week 4 (W4). This difference, however, is more marked, and is significant, if SrEPSC amplitudes are pooled across all neurons within a group (by event; *p < 0.05). D2, Because of the difficulty in obtaining clean SrEPSC recordings in adult tissue, GFP+ cells were treated as one group and compared alongside developing postnatal and adult PGCs. Whether analyzed by cell or by event, no significant differences were observed between PGC groups.
Figure 6.
Paired-pulse depression at ON synapses does not change with postnatal maturation of ETCs and PGCs, nor with maturation of adult-born GFP+ neurons. A, Paired-pulse AMPA responses evoked at varying ISIs at either −70 mV or +40 mV. Responses depressed strongly with a 50 ms ISI; this depression lessened with increasing ISI but was still evident at 5000 ms intervals. Values at each peak show the PPR calculated by dividing the peak amplitude of the second response by that of the first. B, Mean ± SEM. PPR values for all three cell types, over all maturation stages. A one-way ANOVA for responses at +40 mV revealed no significant differences between cell groups, whereas a two-way ANOVA for responses at −70 mV revealed significant differences between ISIs (p < 0.0001), but no significant differences between cell types, consistent with a presynaptic cause of PPR. C, Representative traces at −70 mV, 50 ms ISI for immature and mature stages in all three cell groups. Examples are normalized with respect to their initial peak; values at bottom are PPR. D, No significant correlations of PPR (−70 mV, 50 ms ISI) with maturation in any cell group. r and p report the results of nonparametric rank correlations. A, Adult. E, No significant group differences in PPR (−70 mV, 50 ms ISI) for any cell type. W1, Postnatal week 1; W4, postnatal week 4; 7–14, 45, and 90 refer to GFP+ cell groupings based on dpi.
Figure 7.
Presynaptic modulation of ON input via GABAB receptors is present and mature in the first postnatal week. A, Example traces recorded from PGCs at P5 and P26, showing AMPA responses to paired-pulse stimulation (50 ms ISI, −70 mV). Control traces (gray) show strongly depressing responses before the addition of the GABAB receptor agonist baclofen (0.5 μ
m
). Black traces show responses after baclofen application: initial response amplitude is decreased and PPR becomes less strong, consistent with a decrease in presynaptic release probability. B, Group comparisons show that baclofen's effects on initial response amplitude and PPR are significant in both the first (W1) and the fourth (W4) postnatal weeks (1-sample t test vs 1; *p < 0.05; **p < 0.01; ***p < 0.001), but do not differ significantly between different stages of ON input maturation.
Similar articles
- Microglial depletion disrupts normal functional development of adult-born neurons in the olfactory bulb.
Wallace J, Lord J, Dissing-Olesen L, Stevens B, Murthy VN. Wallace J, et al. Elife. 2020 Mar 9;9:e50531. doi: 10.7554/eLife.50531. Elife. 2020. PMID: 32150529 Free PMC article. - Disruption of Coordinated Presynaptic and Postsynaptic Maturation Underlies the Defects in Hippocampal Synapse Stability and Plasticity in Abl2/Arg-Deficient Mice.
Xiao X, Levy AD, Rosenberg BJ, Higley MJ, Koleske AJ. Xiao X, et al. J Neurosci. 2016 Jun 22;36(25):6778-91. doi: 10.1523/JNEUROSCI.4092-15.2016. J Neurosci. 2016. PMID: 27335408 Free PMC article. - Integration and maturation of newborn neurons in the adult olfactory bulb--from synapses to function.
Nissant A, Pallotto M. Nissant A, et al. Eur J Neurosci. 2011 Mar;33(6):1069-77. doi: 10.1111/j.1460-9568.2011.07605.x. Eur J Neurosci. 2011. PMID: 21395850 Review. - The impact of adult neurogenesis on olfactory bulb circuits and computations.
Lepousez G, Valley MT, Lledo PM. Lepousez G, et al. Annu Rev Physiol. 2013;75:339-63. doi: 10.1146/annurev-physiol-030212-183731. Epub 2012 Nov 26. Annu Rev Physiol. 2013. PMID: 23190074 Review.
Cited by
- Wiring Olfaction: The Cellular and Molecular Mechanisms that Guide the Development of Synaptic Connections from the Nose to the Cortex.
de Castro F. de Castro F. Front Neurosci. 2009 Dec 4;3:52. doi: 10.3389/neuro.22.004.2009. eCollection 2009. Front Neurosci. 2009. PMID: 20582279 Free PMC article. - A Pool of Postnatally Generated Interneurons Persists in an Immature Stage in the Olfactory Bulb.
Benito N, Gaborieau E, Sanz Diez A, Kosar S, Foucault L, Raineteau O, De Saint Jan D. Benito N, et al. J Neurosci. 2018 Nov 14;38(46):9870-9882. doi: 10.1523/JNEUROSCI.1216-18.2018. Epub 2018 Oct 3. J Neurosci. 2018. PMID: 30282727 Free PMC article. - How, when, and where new inhibitory neurons release neurotransmitters in the adult olfactory bulb.
Bardy C, Alonso M, Bouthour W, Lledo PM. Bardy C, et al. J Neurosci. 2010 Dec 15;30(50):17023-34. doi: 10.1523/JNEUROSCI.4543-10.2010. J Neurosci. 2010. PMID: 21159972 Free PMC article. - Using affordable LED arrays for photo-stimulation of neurons.
Valley M, Wagner S, Gallarda BW, Lledo PM. Valley M, et al. J Vis Exp. 2011 Nov 15;(57):3379. doi: 10.3791/3379. J Vis Exp. 2011. PMID: 22127025 Free PMC article. - Early formation of GABAergic synapses governs the development of adult-born neurons in the olfactory bulb.
Pallotto M, Nissant A, Fritschy JM, Rudolph U, Sassoè-Pognetto M, Panzanelli P, Lledo PM. Pallotto M, et al. J Neurosci. 2012 Jun 27;32(26):9103-15. doi: 10.1523/JNEUROSCI.0214-12.2012. J Neurosci. 2012. PMID: 22745509 Free PMC article.
References
- Aroniadou-Anderjaska V, Zhou FM, Priest CA, Ennis M, Shipley MT. Tonic and synaptically evoked presynaptic inhibition of sensory input to the rat olfactory bulb via GABA(B) heteroreceptors. J Neurophysiol. 2000;84:1194–1203. - PubMed
- Bayer SA. 3H-thymidine-radiographic studies of neurogenesis in the rat olfactory bulb. Exp Brain Res. 1983;50:329–340. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources