Rapid activity-dependent modifications in synaptic structure and function require bidirectional Wnt signaling - PubMed (original) (raw)
Comparative Study
Rapid activity-dependent modifications in synaptic structure and function require bidirectional Wnt signaling
Bulent Ataman et al. Neuron. 2008.
Abstract
Activity-dependent modifications in synapse structure play a key role in synaptic development and plasticity, but the signaling mechanisms involved are poorly understood. We demonstrate that glutamatergic Drosophila neuromuscular junctions undergo rapid changes in synaptic structure and function in response to patterned stimulation. These changes, which depend on transcription and translation, include formation of motile presynaptic filopodia, elaboration of undifferentiated varicosities, and potentiation of spontaneous release frequency. Experiments indicate that a bidirectional Wnt/Wg signaling pathway underlies these changes. Evoked activity induces Wnt1/Wg release from synaptic boutons, which stimulates both a postsynaptic DFz2 nuclear import pathway as well as a presynaptic pathway involving GSK-3beta/Shaggy. Our findings suggest that bidirectional Wg signaling operates downstream of synaptic activity to induce modifications in synaptic structure and function. We propose that activation of the postsynaptic Wg pathway is required for the assembly of the postsynaptic apparatus, while activation of the presynaptic Wg pathway regulates cytoskeletal dynamics.
Figures
Figure 1
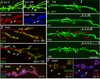
Acute spaced stimulation induces the formation of synaptopods at the NMJ. (A) Time lapse images of live NMJs expressing membrane tethered GFP in motorneurons about 2.5 hr from the beginning of spaced depolarization (exact time is stated in minutes in each panel). Red arrowheads point to a retracting synaptopod. White arrowheads point to elongating synaptopods. (B) High K+-induced depolarization paradigms. (C) Number of synaptopods per NMJ arbor in wild type controls and preparations stimulated with spaced high K+ depolarization. (D) Comparison of a representative NMJ arbor imaged live (D1) before and (D2) after 2.5 hrs from the beginning of spaced high K+ depolarization, showing the increase in synaptopod frequency and length. Left column shows an entire NMJ at muscle 6 and 7, and right column is a high magnification view of the NMJ area circumscribed by the box in the left column panels. Arrows point to synaptopods. ** = p< 0.001. Calibration scale is 4.5 µm in A, 23 µm in D left column, and 5 µm in D right column.
Figure 2
Acute spaced stimulation induces the formation of undifferentiated “ghost boutons”. (A) De novo ghost bouton formation observed live, after 150 min from the beginning of spaced depolarization. In A (bottom row) the same sample shown in (A top right panel) is exhibited after fixation and immunocytochemical staining with anti-HRP (left panel) and anti-DLG (right panel) antibodies, Arrows point to two ghost boutons in the live and fixed preparations. (B, C) NMJs double stained with anti-HRP and anti-DLG antibodies in (B) a control unstimulated preparation, and (C) a sample subjected to spaced 5X K+ depolarization, fixed and imaged at 2.5 hrs after dissection. Arrows in (C) point to ghost boutons, which are recognized by labeling with anti-HRP antibodies, while lacking DLG immunoreactivity. (D, F) View of ghost boutons (arrows) in preparations fixed after stimulation and triple stained with (D) HRP, DLG, and CSP antibodies showing that ghost boutons contain a synaptic vesicle marker, and (F) HRP, NC82, and GluRIII antibodies showing that ghost boutons are devoid of GluR clusters and most active zones. (E) Time-lapse image series in which the preparation was imaged before stimulation and at the indicated times during the spaced stimulation protocol (arrowheads). Note the sudden appearance of ghost boutons (red arrows) after the 4th depolarization pulse. Calibration scale is 12 µm in (A); 17 µm in (B, C); 6.5 µm in (D, F); and 24 µm in (E).
Figure 3
Ghost boutons form de novo in intact undissected larvae and develop into mature boutons by acquiring postsynaptic GluR and presynaptic Brp clusters. Time-lapse imaging through the intact cuticle of NMJs expressing presynaptic myr-RFP (red) and either postsynaptic GluR-GFP (green) or presynaptic Brp-GFP (green) showing (A) de novo formation of a ghost bouton (arrows) in muscle 27 at 24hr and clustering of GluR receptors on the ghost bouton (arrows) at 36hr. (B) Progressive increase in the number of GluR clusters (arrows) in a differentiating bouton, on muscle 27, over a 24hr period. Arrowhead in myr-RFP at 0 hr points to a synaptopod (C) Another example of a ghost bouton (arrows) in muscles 14 and 30 imaged at 0hr, which acquired GluR clusters (arrows) at 24 hours. * = peptidergic ending, which normally lacks GluR clusters. (D, E) Ghost boutons (arrows) in muscles 14 and 30 at 0hr, which acquired Brp clusters at 18 hr (arrows). Thin neurites may appear invisible, next to bright mRFP signal of boutons. Calibration scale is 5 µm in A, 3 µm in B, and 8 µm in C–E.
Figure 4
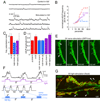
Activity-dependent ghost bouton formation depends on spaced stimulation, Ca++, as well as transcription and translation. (A–C) Number of ghost boutons upon K+ depolarization induced (A) by using alternative stimulation protocols, (B) in the presence of different drugs and Ca++ conditions (no Ca++ and 0.5 mM EGTA) and (C) in parats mutants. *= p<0.05, **= p<0.001, ***=p<0.0001.
Figure 5
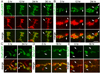
Potentiation of spontaneous release frequency after spaced K+ depolarization, nerve stimulation, and light induced stimulation of motorneurons by using ChR2. (A) mEJP traces in (top) controls and (bottom) samples subjected to spaced 5X K+ depolarization. (B) Normalized probability distribution of mEJP frequencies, obtained by dividing the mEJP frequency of experimental samples by the mean control frequencies, after spaced 5X K+ depolarization (red), 5X nerve stimulation (blue), or 5X light stimulation of presynaptic ChR2 (purple). (C) mEJP frequency potentiation index (mEJP frequency in experimental samples divided by the mean control frequency). (D) Mean mEJP amplitude after the above stimulation paradigms. (F) Paradigms for nerve and light stimulation and postsynaptic recording of responses to the 5X light ChR2 paradigm. Traces below the recording correspond to (purple) LED lights on and off cycles and (blue) nerve stimulation, with the entire 5X paradigm shown in black. (E, G) Morphological plasticity of NMJs upon (E) 5X nerve and (G) 5X ChR2 stimulation. (E) shows an instance of extending and retracting synaptopods (arrows mark moving synaptopods, white lines are fiduciary markers) in a presynaptic mCD8-GFP-labeled preparation imaged live. (G) shows an example of enhanced ghost bouton formation (arrows) in a fixed preparation double labeled with anti-HRP (green) and anti-DLG (red) antibodies. *= p<0.05, **=p<0.001. Calibration scale is 3 µm in E and 6 µm in G.
Figure 6
Wg signaling regulates ghost bouton formation and mEJP potentiation. (A) Number of ghost boutons after 0X, 3X, or 5X spaced K+ stimulation in controls (red), _wgts/_+ heterozygous and wg1 homozygous mutants (blue), upon expressing UAS-Wg in motorneurons (orange), and upon rescuing wgts with UAS-Wg in motorneurons (green). (B) Normalized probability distribution of mEJP frequencies in the indicated genotypes after spaced 5X K+ stimulation. Normalization was obtained by dividing the frequency of experimental samples by the mean control frequencies. (C) Frequency potentiation index. (D) Mean mEJP amplitude in the indicated genotypes in controls and samples subjected to spaced 5X K+ stimulation. *= p<0.05, **= p<0.001, ***=p<0.0001.
Figure 7
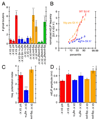
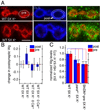
Activity-dependent Wg secretion by synaptic boutons. (A) Wg immunoreactivity (green) at synaptic boutons of wild type (top row) controls and (bottom row) specimens subjected to spaced 5X K+ depolarization in samples triple stained with anti-HRP (red), anti-DLG (blue) and anti-Wg (green). Images correspond to single confocal slices. The postsynaptic (DLG minus HRP) and the presynaptic (HRP) areas are outlined in white in the middle-upper and the left-upper rows respectively. (B) Pre- (red) and postsynaptic (blue) Wg levels in wild type controls and samples subjected to spaced 5X K+ depolarization, in the presence or absence of Ca++. Numbers in the Y-axis correspond to the difference in mean Wg intensity levels between control and experimental samples. (C) Pre- (red) and postsynaptic (blue) Wg levels in response to 5X K+ depolarization after blocking activity with parats1 and ShiDNts-pre. Wg levels were normalized by dividing the Wg levels after the 5X K+ paradigm at restrictive temperature (HS) by the Wg levels after the 5X K+ paradigm at permissive temperature (RT). Calibration scale is 2.5 µm in A.
Figure 8
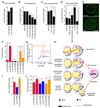
Activity-dependent regulation of postsynaptic DFz2C nuclear import and role of Sgg in rapid activity-dependent changes at the NMJ. (A–D) Number of DFz2C nuclear spots in (A) wild type after spaced 5X K+ depolarization, (B) larvae expressing ShiDNts in motorneurons and in which neurotransmitter release was blocked for 90, 150, and 450 min, (C) parats1 animals in which action potentials were blocked for 150 min, and (D) eag Sh mutants (chronic hyperexcitability) and larvae expressing ChR2 in motorneurons and stimulated by light with a developmental paradigm (see Methods). (E) Nuclear DFz2C immunoreactivity in the postsynaptic muscle nucleus of wild type (top) and eag Sh mutants. White circles outline the nucleus. (F, G, I, J) Effect of alterations in Sgg activity in ghost bouton number and mEJP potentiation. (F) Number of ghost boutons, (I) mEJPs frequency potentiation index and (J) and mean mEJP amplitude upon spaced 5X K+ stimulation in controls (red) as well as in animals overexpressing Sgg (blue) and SggDN (orange) in motorneurons. (G) shows the normalized probability distribution of mEJP frequencies in the above genotypes. *= p<0.05, **= p<0.001, ***=p<0.0001. Calibration scale in E is 11 µm. (G) Proposed model for activity-dependent regulation of synapse formation at the NMJ. Patterned activity induces Wg secretion from presynaptic terminals. Once released, Wg binds to DFz2 receptors localized both pre- and postsynaptically. In the presynaptic cell, Wg transduction leads to the formation of synaptopods and ghost boutons in part through regulation of cytoskeletal dynamics, which involves inhibition of GSK-3β/Sgg activity. In the postsynaptic cell, Wg activates the Frizzled Nuclear Import (FNI) pathway and signals the formation/stabilization of synaptic specializations through transcriptional regulation.
Similar articles
- Wnt signaling during synaptic development and plasticity.
Budnik V, Salinas PC. Budnik V, et al. Curr Opin Neurobiol. 2011 Feb;21(1):151-9. doi: 10.1016/j.conb.2010.12.002. Epub 2011 Jan 14. Curr Opin Neurobiol. 2011. PMID: 21239163 Free PMC article. Review. - Presynaptic local signaling by a canonical wingless pathway regulates development of the Drosophila neuromuscular junction.
Miech C, Pauer HU, He X, Schwarz TL. Miech C, et al. J Neurosci. 2008 Oct 22;28(43):10875-84. doi: 10.1523/JNEUROSCI.0164-08.2008. J Neurosci. 2008. PMID: 18945895 Free PMC article. - Cortactin Is a Regulator of Activity-Dependent Synaptic Plasticity Controlled by Wingless.
Alicea D, Perez M, Maldonado C, Dominicci-Cotto C, Marie B. Alicea D, et al. J Neurosci. 2017 Feb 22;37(8):2203-2215. doi: 10.1523/JNEUROSCI.1375-16.2017. Epub 2017 Jan 25. J Neurosci. 2017. PMID: 28123080 Free PMC article. - Perlecan regulates bidirectional Wnt signaling at the Drosophila neuromuscular junction.
Kamimura K, Ueno K, Nakagawa J, Hamada R, Saitoe M, Maeda N. Kamimura K, et al. J Cell Biol. 2013 Jan 21;200(2):219-33. doi: 10.1083/jcb.201207036. Epub 2013 Jan 14. J Cell Biol. 2013. PMID: 23319599 Free PMC article. - WNTs tune up the neuromuscular junction.
Korkut C, Budnik V. Korkut C, et al. Nat Rev Neurosci. 2009 Sep;10(9):627-34. doi: 10.1038/nrn2681. Nat Rev Neurosci. 2009. PMID: 19693027 Free PMC article. Review.
Cited by
- Inhibition of GSK3α,β rescues cognitive phenotypes in a preclinical mouse model of CTNNB1 syndrome.
Alexander JM, Vazquez-Ramirez L, Lin C, Antonoudiou P, Maguire J, Wagner F, Jacob MH. Alexander JM, et al. EMBO Mol Med. 2024 Sep;16(9):2109-2131. doi: 10.1038/s44321-024-00110-5. Epub 2024 Aug 5. EMBO Mol Med. 2024. PMID: 39103699 Free PMC article. - γ-secretase promotes Drosophila postsynaptic development through the cleavage of a Wnt receptor.
Restrepo LJ, DePew AT, Moese ER, Tymanskyj SR, Parisi MJ, Aimino MA, Duhart JC, Fei H, Mosca TJ. Restrepo LJ, et al. Dev Cell. 2022 Jul 11;57(13):1643-1660.e7. doi: 10.1016/j.devcel.2022.05.006. Epub 2022 Jun 1. Dev Cell. 2022. PMID: 35654038 Free PMC article. - Dendritic growth gated by a steroid hormone receptor underlies increases in activity in the developing Drosophila locomotor system.
Zwart MF, Randlett O, Evers JF, Landgraf M. Zwart MF, et al. Proc Natl Acad Sci U S A. 2013 Oct 1;110(40):E3878-87. doi: 10.1073/pnas.1311711110. Epub 2013 Sep 16. Proc Natl Acad Sci U S A. 2013. PMID: 24043825 Free PMC article. - Wnt signaling during synaptic development and plasticity.
Budnik V, Salinas PC. Budnik V, et al. Curr Opin Neurobiol. 2011 Feb;21(1):151-9. doi: 10.1016/j.conb.2010.12.002. Epub 2011 Jan 14. Curr Opin Neurobiol. 2011. PMID: 21239163 Free PMC article. Review. - A comparison of three different methods of eliciting rapid activity-dependent synaptic plasticity at the Drosophila NMJ.
Maldonado-Díaz C, Vazquez M, Marie B. Maldonado-Díaz C, et al. PLoS One. 2021 Nov 30;16(11):e0260553. doi: 10.1371/journal.pone.0260553. eCollection 2021. PLoS One. 2021. PMID: 34847197 Free PMC article.
References
- Atwood HL, Govind CK, Wu CF. Differential ultrastructure of synaptic terminals on ventral longitudinal abdominal muscles in Drosophila larvae. J Neurobiol. 1993;24:1008–1024. - PubMed
- Biederer T, Sara Y, Mozhayeva M, Atasoy D, Liu X, Kavalali ET, Sudhof TC. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science. 2002;297:1525–1531. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 MH070000/MH/NIMH NIH HHS/United States
- R01 MH070000/MH/NIMH NIH HHS/United States
- DK32520/DK/NIDDK NIH HHS/United States
- MH070000/MH/NIMH NIH HHS/United States
- R01 MH070000-01/MH/NIMH NIH HHS/United States
- P30 DK032520/DK/NIDDK NIH HHS/United States
- R01 MH070000-04/MH/NIMH NIH HHS/United States
- R01 MH070000-03/MH/NIMH NIH HHS/United States
- R01 MH070000-02/MH/NIMH NIH HHS/United States
- R01 MH070000-05/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases