The ubiquitin-editing enzyme A20 restricts nucleotide-binding oligomerization domain containing 2-triggered signals - PubMed (original) (raw)
doi: 10.1016/j.immuni.2008.02.002.
Regina-Celeste Ahmad, Rita Tavares, Min Wang, Dana Philpott, Emre E Turer, Bettina L Lee, Nataliya Shiffin, Rommel Advincula, Barbara A Malynn, Catherine Werts, Averil Ma
Affiliations
- PMID: 18342009
- PMCID: PMC3606373
- DOI: 10.1016/j.immuni.2008.02.002
The ubiquitin-editing enzyme A20 restricts nucleotide-binding oligomerization domain containing 2-triggered signals
Osamu Hitotsumatsu et al. Immunity. 2008 Mar.
Abstract
Muramyl dipeptide (MDP), a product of bacterial cell-wall peptidoglycan, activates innate immune cells by stimulating nucleotide-binding oligomerization domain containing 2 (NOD2) -dependent activation of the transcription factor NFkappaB and transcription of proinflammatory genes. A20 is a ubiquitin-modifying enzyme that restricts tumor necrosis factor (TNF) receptor and Toll-like receptor (TLR) -induced signals. We now show that MDP induces ubiquitylation of receptor- interacting protein 2 (RIP2) in primary macrophages. A20-deficient cells exhibit dramatically amplified responses to MDP, including increased RIP2 ubiquitylation, prolonged NFkappaB signaling, and increased production of proinflammatory cytokines. In addition, in vivo responses to MDP are exaggerated in A20-deficient mice and in chimeric mice bearing A20-deficient hematopoietic cells. These exaggerated responses occur independently of the TLR adaptors MyD88 and TRIF as well as TNF signals. These findings indicate that A20 directly restricts NOD2 induced signals in vitro and in vivo, and provide new insights into how these signals are physiologically restricted.
Figures
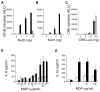
Figure 1. A20 Inhibits MDP- and DAP-Dependent NFκB Transcriptional Activity
(A–C) NFκB-dependent luciferase reporter assays of NOD2 or NOD1 transfected 293T cells. Indicated amounts of (A) NOD2, (B) NOD1, or (C) cAMP response element (CRE) plasmid trans-fected 293T cells were stimulated with MDP, Tri-DAP, or forskolin, respectively. Cells were also cotransfected with either 0 ng (black columns), 5 ng (gray columns), or 25 ng (white columns) of an A20 expression plasmid. Note the induction of NOD2-mediated NFκB transcriptional activity by MDP, the induction of NOD1-mediated NFκB by Tri-DAP, and the dose-dependent inhibition of these inductions by A20. By contrast, forskolin induced cAMP response element driven luciferase activity is not suppressed by A20. Data are reported in relative luciferase units (RLU) and are representative of three independent experiments. (D and E) A20 is required for restricting MDP induced responses. ELISA analyses of secreted IL-1β and IL-6 from BMDCs. BMDCs from Tnfaip3+/+ (white columns) and _Tnfaip3_−/− (black columns) mice were stimulated with the indicated doses of MDP for 12, 24, or 36 hr (for IL-6 analyses) or 24 hr (for IL-1β analyses), after which supernatants were harvested and analyzed by ELISA for (D) IL-6 and (E) IL-1β secretion. Data are representative of three independent experiments, with standard deviation (bars) indicated.
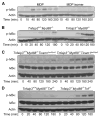
Figure 2. A20 Is Required for Restricting MDP-Induced Signaling Activity
(A) Immunoblotting analyses of phospho-IκBα, IκBα, A20, NOD2, RIP2, and actin protein expression in _Tnfaip3_−/− and Tnfaip3+/+ BMDMs after MDP stimulation. _Tnfaip3_−/− and Tnfaip3+/+ BMDMs were stimulated with 10 μg/ml of MDP, and cell lysates were harvested at the indicated time points for immunoblotting analysis. Note increased phospho-IκBα protein expression in _Tnfaip3_−/− cells at later time points, indicating prolonged NFκB-signaling activity. Note similar amounts of IκBα, NOD2, and RIP2 in Tnfaip3+/+ and _Tnfaip3_−/− cells after MDP stimulation. Note constant A20 protein expression in Tnfaip3+/+ cells (and not in _Tnfaip3_−/− cells) after MDP stimulation. Actin protein expression is shown as a control. (B) IKK kinase assay of _Tnfaip3_−/− and Tnfaip3+/+ BMDMs after MDP stimulation. _Tnfaip3_−/− and Tnfaip3+/+ BMDMs were stimulated with MDP, and cell ly-sates were harvested at the indicated time points, immunoprecipitated with anti-IKKγ antibody, incubated with GST-IκBα substrate, and analyzed by immunoblotting for phospho-GST-IκBα levels. Note increased phospho-GST-IκBα expression in _Tnfaip3_−/− BMDMs indicating prolonged IKK kinase activity. IKKβ protein expression in the immunoprecipitates are shown as a control.
Figure 3. A20 Directly Regulates MDP-Induced NFκB Signaling
(A) Immunoblotting analyses of MDP versus MDP isomer-stimulated BMDMs. Wild-type BMDMs were stimulated with either MDP or an MDP isomer from the same commercial source that does not activate NOD2 for the indicated time points, after which lysates were analyzed for expression of phospho-IκBα. Note that the MDP isomer fails to elicit NFκB signaling in BMDMs. (B) Immunoblotting analyses of phospho-IκBα protein levels in _Tnfaip3_−/− _Myd88_−/− and Tnfaip3+/+ _Myd88_−/− BMDMs after MDP stimulation. _Tnfaip3_−/− _Myd88_−/− and Tnfaip3+/+ _Myd88_−/− BMDMs were stimulated with 10 μg/ml of MDP, and cell lysates were harvested at the indicated time points for immunoblotting analysis. Note that increased phospho-IκBα protein expression is observed in _Tnfaip3_−/− _Myd88_−/− cells at later time points, indicating that A20 restricts MDP NFκB-signaling activity independently of MyD88. (C) Immunoblotting analyses of phospho-IκBα protein levels in _Tnfaip3_−/− _Myd88_−/− Ticam1lps2/lps2 and Tnfaip3+/+ _Myd88_−/− Ticam1lps2/lps2 BMDMs after MDP stimulation. _Tnfaip3_−/− _Myd88_−/− Ticam1lps2/lps2 and Tnfaip3+/+ _Myd88_−/− Ticam1lps2/lps2 BMDMs were stimulated with 10 μg/ml of MDP, and cell lysates were harvested at the indicated time points for immunoblotting analysis of phospho-IκBα and IκBα. Note that increased phospho-IκBα protein levels are observed in _Tnfaip3_−/− _Myd88_−/− Ticam1lps2/lps2 BMDMs compared with Tnfaip3+/+ _Myd88_−/− Ticam1lps2/lps2 cells. (D) Immunoblotting analyses of phospho-IκBα protein levels in _Tnfaip3_−/− _Myd88_−/− _Tnf_−/− and Tnfaip3+/+ _Myd88_−/− _Tnf_−/− BMDMs after MDP stimulation. _Tnfaip3_−/− _Myd88_−/− _Tnf_−/− and Tnfaip3+/+ _Myd88_−/− _Tnf_−/− BMDMs were stimulated with 10 μg/ml of MDP, and cell lysates were harvested at the indicated time points for immunoblotting analysis of phospho-IκBα and IκBα proteins. Note that increased phospho-IκBα protein levels are observed in _Tnfaip3_−/− _Myd88_−/− _Tnf_−/− BMDMs compared with Tnfaip3+/+ _Myd88_−/− _Tnf_−/− cells. Immunoblots for actin expression are shown as controls for all experiments. All data are representative of at least three independent experiments.
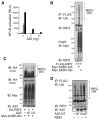
Figure 4. A20 Deubiquitylates RIP2 and Restricts RIP2-Dependent NFκB Activity
(A) NFκB-dependent luciferase reporter assays of RIP2-transfected 293T cells. 293T cells were transfected with either 0 ng (white columns), 5 ng (light gray columns), 25 ng (dark gray columns), or 125 ng (black columns) of RIP2 expression plasmid and the indicated amounts of A20 expression plasmids. (White columns are essentially at baseline.) Cell lysates were analyzed for luciferase activity. Note the dose-dependent induction of NFκB transcriptional activity by RIP2 and the dose-dependent inhibition of this induction by A20. Data are reported in relative luciferase units and are representative of three independent experiments. (B) Immunoblotting assay of RIP2 ubiquitylation in cells. FLAG-tagged RIP2 and Myc-tagged ubiquitin were cotransfected into 293T cells, after which cells were lysed in NP-40 buffer, and boiled in 1% SDS. Samples were then diluted 1:10 with PBS, immunoprecipitated with FLAG-specific antibody (RIP2), and immunoblotted for the presence of Myc-ubiquitin. Note that RIP2 is preferentially ubiquitylated with K48R ubiquitin compared to K63R ubiquitin in cells (compare second and third lanes of top panel). Immunoblotting of Myc-ubiquitin proteins in preimmunoprecipitates are shown in bottom panel as controls. (C) A20 restricts RIP2 ubiquitylation in cells. Immunoblotting analyses of RIP2 ubiquitylation with A20 cotransfection. HA-RIP2, Myc-K48R ubiquitin, and either wild-type or mutant C103A A20 were cotransfected into 293T cells, after which lysates were immunoblotted for Myc (ubiquitin). Note that cotrans-fected wild-type A20 but not C103A mutant A20 reduces RIP2 ubiquitylation in cells (top panels). Immunoblotting of Myc-ubiquitin proteins is shown in bottom panel as controls. (D) Recombinant A20 protein deubiquitylates RIP2 in a cell-free assay. Immu-noblotting analyses of RIP2 ubiquitylation after cell-free deubiquitylation. FLAG-RIP2 and myc-K48R mutant ubiquitin were cotransfected into 293T cells, after which cells were lysed in NP-40 lysis buffer. Samples were then immunoprecipitated with FLAG specific antibody (RIP2), washed, and incubated with bacterial recombinant N-terminal A20 protein or mutant C103A A20 protein for 90 min. Wild-type N-terminal A20 protein was also incubated with ubiquitylated RIP2 in the presence of the cysteine protease inhibitor N-ethyl maleimide (NEM). Reactions were then analyzed by immunoblotting for (Myc) ubiquitin. Note that wild-type A20 but not mutant C103A A20 protein reduces RIP2 ubiquitylation (compare second and third lanes). Note also that A20-mediated reduction of RIP2 ubiquitylation is inhibited by NEM (compare second and fourth lanes). Data are representative of three independent experiments.
Figure 5. A20 Is Required for Restricting Endogenous RIP2 and Not TRAF6 Ubiquitylation after MDP Stimulation
(A) Immunoblotting analysis of endogenous ubiquitylated RIP2 in BMDMs after MDP stimulation. _Tnfaip3_−/− and Tnfaip3+/+ BMDMs were stimulated with 10 μg/ml MDP and lysed in RIPA lysis buffer at the indicated time points, boiled in 1%SDS, diluted 1:10 with RIPA buffer, and immunoprecipitated with anti-RIP2 antibody. Immunoprecipitated RIP2 was then analyzed by immuno-blotting for ubiquitin. Note the presence of prolonged RIP2 ubiquitylation in _Tnfaip3_−/− BMDMs after MDP stimulation. Immunoblotting of RIP2 immuno-precipitates is shown below as a control. (B) Immunoblotting analysis of endogenous ubiquitylated TRAF6 in BMDMs after MDP stimulation. _Tnfaip3_−/− and Tnfaip3+/+ BMDMs were stimulated as in (A) above and immunoprecipitated with anti-TRAF6 antibody. Immuno-precipitated TRAF6 was then analyzed by immunoblotting for ubiquitin. Note similar TRAF6 ubiquitylation in Tnfaip3+/+ and _Tnfaip3_−/− BMDMs after MDP stimulation. Immunoprecipitates using antibody-coated beads only (no lysates) are shown as controls in right lane of each immunoblot. Immunoblot-ting for TRAF6 on TRAF6 immunoprecipitates is shown below as a control. Data are representative of seven independent experiments.
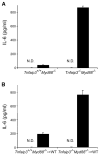
Figure 6. A20 Restricts MDP-Induced Inflammatory Responses In Vivo
(A and B) ELISA analyses of serum from MDP-injected mice. Twenty-five milligrams over kilograms of MDP (or H2O) was injected into (A) intact Tnfaip3+/+ _Myd88_−/− and _Tnfaip3_−/− _Myd88_−/− mice, and (B) chimeric mice reconstituted with HSCs from Tnfaip3+/+ _Myd88_−/− and _Tnfaip3_−/− _Myd88_−/− mice. Serum was harvested 4 hr after MDP injection and analyzed for IL-6 levels by ELISA. White columns indicate samples from mice injected with H2O, and indicate that no IL-6 above baseline was detected (ND). Black columns indicate samples from MDP-injected mice. Note increased levels of serum IL-6 in (A) intact _Tnfaip3_−/− _Myd88_−/− mice compared with Tnfaip3+/+ _Myd88_−/− mice and in (B) chimeric mice bearing _Tnfaip3_−/− _Myd88_−/− HSCs compared with those bearing Tnfaip3+/+ _Myd88_−/− cells. Data were obtained from three sets of paired mice.
Similar articles
- NOD2 pathway activation by MDP or Mycobacterium tuberculosis infection involves the stable polyubiquitination of Rip2.
Yang Y, Yin C, Pandey A, Abbott D, Sassetti C, Kelliher MA. Yang Y, et al. J Biol Chem. 2007 Dec 14;282(50):36223-9. doi: 10.1074/jbc.M703079200. Epub 2007 Oct 18. J Biol Chem. 2007. PMID: 17947236 - Nod2 and Rip2 contribute to innate immune responses in mouse neutrophils.
Jeong YJ, Kang MJ, Lee SJ, Kim CH, Kim JC, Kim TH, Kim DJ, Kim D, Núñez G, Park JH. Jeong YJ, et al. Immunology. 2014 Oct;143(2):269-76. doi: 10.1111/imm.12307. Immunology. 2014. PMID: 24766550 Free PMC article. - TNF-α-Induced NOD2 and RIP2 Contribute to the Up-Regulation of Cytokines Induced by MDP in Monocytic THP-1 Cells.
Chen X, Xiao Z, Xie X, Liu X, Jiang M, Yuan C, Yang L, Hu J. Chen X, et al. J Cell Biochem. 2018 Jul;119(7):5072-5081. doi: 10.1002/jcb.26227. Epub 2018 Mar 25. J Cell Biochem. 2018. PMID: 28639322 - [Bone destruction caused by osteoclasts].
Yamashita T, Takahashi N, Yang S, Sato N, Udagawa N. Yamashita T, et al. Clin Calcium. 2006 Feb;16(2):234-40. Clin Calcium. 2006. PMID: 16465024 Review. Japanese. - A20: from ubiquitin editing to tumour suppression.
Hymowitz SG, Wertz IE. Hymowitz SG, et al. Nat Rev Cancer. 2010 May;10(5):332-41. doi: 10.1038/nrc2775. Epub 2010 Apr 12. Nat Rev Cancer. 2010. PMID: 20383180 Review.
Cited by
- Chronic ingestion of alcohol modulates expression of ubiquitin editing enzyme A20 in lung macrophages.
Huang QY, Chen YC, Liu SP. Huang QY, et al. Multidiscip Respir Med. 2011 Dec 20;6(6):364-70. doi: 10.1186/2049-6958-6-6-364. Multidiscip Respir Med. 2011. PMID: 22958952 Free PMC article. - A20 (TNFAIP3) alleviates CVB3-induced myocarditis via inhibiting NF-κB signaling.
Gui J, Yue Y, Chen R, Xu W, Xiong S. Gui J, et al. PLoS One. 2012;7(9):e46515. doi: 10.1371/journal.pone.0046515. Epub 2012 Sep 28. PLoS One. 2012. PMID: 23029542 Free PMC article. - A20: linking a complex regulator of ubiquitylation to immunity and human disease.
Ma A, Malynn BA. Ma A, et al. Nat Rev Immunol. 2012 Nov;12(11):774-85. doi: 10.1038/nri3313. Epub 2012 Oct 12. Nat Rev Immunol. 2012. PMID: 23059429 Free PMC article. Review. - Ablation of gly96/immediate early gene-X1 (gly96/iex-1) aggravates DSS-induced colitis in mice: role for gly96/iex-1 in the regulation of NF-kappaB.
Sina C, Arlt A, Gavrilova O, Midtling E, Kruse ML, Müerköster SS, Kumar R, Fölsch UR, Schreiber S, Rosenstiel P, Schäfer H. Sina C, et al. Inflamm Bowel Dis. 2010 Feb;16(2):320-331. doi: 10.1002/ibd.21066. Inflamm Bowel Dis. 2010. PMID: 19714745 Free PMC article. - Ubiquitin becomes ubiquitous in cancer: emerging roles of ubiquitin ligases and deubiquitinases in tumorigenesis and as therapeutic targets.
Shi D, Grossman SR. Shi D, et al. Cancer Biol Ther. 2010 Oct 15;10(8):737-47. doi: 10.4161/cbt.10.8.13417. Epub 2010 Oct 15. Cancer Biol Ther. 2010. PMID: 20930542 Free PMC article. Review.
References
- Abbott DW, Wilkins A, Asara JM, Cantley LC. The Crohn’s disease protein, NOD2, requires RIP2 in order to induce ubiquitinylation of a novel site on NEMO. Curr Biol. 2004;14:2217–2227. - PubMed
- Akira S, Takeda K. Toll-like receptor signaling. Nat Rev Immunol. 2004;4:499–511. - PubMed
- Boone DL, Turer EE, Lee EG, Ahmad RC, Wheeler MT, Tsui C, Hurley P, Chien M, Chai S, Hitotsumatsu O, et al. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat Immunol. 2004;5:1052–1060. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases