NAD synthase NMNAT acts as a chaperone to protect against neurodegeneration - PubMed (original) (raw)
. 2008 Apr 17;452(7189):887-91.
doi: 10.1038/nature06721. Epub 2008 Mar 16.
Affiliations
- PMID: 18344983
- PMCID: PMC3150538
- DOI: 10.1038/nature06721
NAD synthase NMNAT acts as a chaperone to protect against neurodegeneration
R Grace Zhai et al. Nature. 2008.
Abstract
Neurodegeneration can be triggered by genetic or environmental factors. Although the precise cause is often unknown, many neurodegenerative diseases share common features such as protein aggregation and age dependence. Recent studies in Drosophila have uncovered protective effects of NAD synthase nicotinamide mononucleotide adenylyltransferase (NMNAT) against activity-induced neurodegeneration and injury-induced axonal degeneration. Here we show that NMNAT overexpression can also protect against spinocerebellar ataxia 1 (SCA1)-induced neurodegeneration, suggesting a general neuroprotective function of NMNAT. It protects against neurodegeneration partly through a proteasome-mediated pathway in a manner similar to heat-shock protein 70 (Hsp70). NMNAT displays chaperone function both in biochemical assays and cultured cells, and it shares significant structural similarity with known chaperones. Furthermore, it is upregulated in the brain upon overexpression of poly-glutamine expanded protein and recruited with the chaperone Hsp70 into protein aggregates. Our results implicate NMNAT as a stress-response protein that acts as a chaperone for neuronal maintenance and protection. Our studies provide an entry point for understanding how normal neurons maintain activity, and offer clues for the common mechanisms underlying different neurodegenerative conditions.
Figures
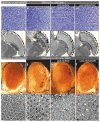
Figure 1. NMNAT suppresses toxicity induced by ataxin-1
a–h, Ommatidial morphology of cross section (a–d) and horizontal section (e–h) of 15-day-old control fly (a, e), and flies overexpressing dAtx-1 (b–d, f–h). Overexpression of wild-type NMNAT (c, g) or enzyme-inactive NMNAT-WR (d, h) restores the rhabdomere degeneration phenotype. i–p, Eye exterior morphology and ommatidial morphology of two-day-old control fly (i, m), and flies overexpressing hAtx-1[82Q] (j–l, n–p). Overexpression of hAtx-1[82Q] leads to rough eye (j) and reduced rhabdomere size (n) phenotypes that are partly suppressed by co-expression of NMNAT (k, o) or NMNAT-WR (l, p).
Figure 2. NMNAT acts as a chaperone in cultured cells
a–c, Cos7 cells are co-transfected with hAtx-1[82Q]-GFP (green) and NMNAT (red). TOTO3 staining (blue) marks nuclei. Scale bar in a for a–c, 20 μm. d, Quantification of GFP fluorescence. Aggregates are defined as objects at least 0.4 μm2 and 3,600 intensity units. Error bars, s.e.m. *P < 0.05, **P < 0.01, ***P < 0.005 (analysis of variance post-hoc test). e–h, Western analysis (e, f) and quantification (g, h) of detergent-insoluble fractions of cells transfected with hAtx-1[82Q]-GFP (e) or hAtx-1[2Q]-GFP (f) and vector, NMNAT or Hsp70, and treated with either DMSO or 10 μM MG132. The level of insoluble hAtx-1[82Q] (g) or hAtx-1[2Q] (h) in the DMSO-treated cells is equal to 1 (fold). Error bars, s.e.m.; n = 4.
Figure 3. Endogenous and overexpressed NMNAT proteins are recruited with Hsp70 into the hAtx-1[82Q] aggregates
a–e, Seven-day-old adult fly brains were stained for NMNAT (green; f–j), hAtx-1[82Q] (red; k–o) and Hsp70 (blue; p–t). Higher magnifications of boxed areas are shown in u–y. Endogenous NMNAT is primarily nuclear (f). hAtx-1[82Q] forms nuclear aggregates (l). Endogenous NMNAT is upregulated and recruited to the aggregates (g). NMNAT is distributed to the cytoplasm when singly overexpressed (h), but is recruited to ataxin aggregates when co-expressed with hAtx-1[82Q] (i, n). Enzymatically inactive NMNAT-WR is also recruited into ataxin aggregates when co-overexpressed with hAtx-1[82Q] (j, o). Hsp70 is upregulated and recruited into the aggregates (q, s, t).
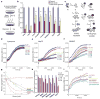
Figure 4. In vivo and in vitro chaperone activity assays
a, b, Luciferase activity was measured without heat shock as controls (blue columns), after 15 min heat shock at 45 °C (red columns) or after 3 h recovery at room temperature (yellow columns). The luciferase activities relative to the controls are displayed. Error bars, s.e.m. *P <0.05, **P <0.01; (analysis of variance post-hoc test); n = 4, triplicate sampling. c, Protein aggregation and its inhibition by chaperones. d–h, Aggregation of citrate synthase is prevented by addition of Hsp70 (e, g), NMNAT (f, g) or human hsNMNAT3 (g) but not by lysozyme (d). g, The relative aggregation rate is calculated by the change in absorbance per minute and the aggregation rate of CS alone is set to 1. (h) The percentage of remaining aggregation is calculated at the saturation point (8000 s). i, Insulin aggregation is inhibited by Hsp70, NMNAT, NMNAT-WR or hsNMNAT3 but not BSA or lysozyme.
Similar articles
- NMNAT: It's an NAD+ synthase… It's a chaperone… It's a neuroprotector.
Brazill JM, Li C, Zhu Y, Zhai RG. Brazill JM, et al. Curr Opin Genet Dev. 2017 Jun;44:156-162. doi: 10.1016/j.gde.2017.03.014. Epub 2017 Apr 23. Curr Opin Genet Dev. 2017. PMID: 28445802 Free PMC article. Review. - Molecular chaperones protect against JNK- and Nmnat-regulated axon degeneration in Drosophila.
Rallis A, Lu B, Ng J. Rallis A, et al. J Cell Sci. 2013 Feb 1;126(Pt 3):838-49. doi: 10.1242/jcs.117259. Epub 2012 Dec 21. J Cell Sci. 2013. PMID: 23264732 Free PMC article. - Nmnat restores neuronal integrity by neutralizing mutant Huntingtin aggregate-induced progressive toxicity.
Zhu Y, Li C, Tao X, Brazill JM, Park J, Diaz-Perez Z, Zhai RG. Zhu Y, et al. Proc Natl Acad Sci U S A. 2019 Sep 17;116(38):19165-19175. doi: 10.1073/pnas.1904563116. Epub 2019 Sep 4. Proc Natl Acad Sci U S A. 2019. PMID: 31484760 Free PMC article. - CHIP protects from the neurotoxicity of expanded and wild-type ataxin-1 and promotes their ubiquitination and degradation.
Al-Ramahi I, Lam YC, Chen HK, de Gouyon B, Zhang M, Pérez AM, Branco J, de Haro M, Patterson C, Zoghbi HY, Botas J. Al-Ramahi I, et al. J Biol Chem. 2006 Sep 8;281(36):26714-24. doi: 10.1074/jbc.M601603200. Epub 2006 Jul 10. J Biol Chem. 2006. PMID: 16831871 - Progress in pathogenesis studies of spinocerebellar ataxia type 1.
Cummings CJ, Orr HT, Zoghbi HY. Cummings CJ, et al. Philos Trans R Soc Lond B Biol Sci. 1999 Jun 29;354(1386):1079-81. doi: 10.1098/rstb.1999.0462. Philos Trans R Soc Lond B Biol Sci. 1999. PMID: 10434309 Free PMC article. Review.
Cited by
- Mislocalization of neuronal mitochondria reveals regulation of Wallerian degeneration and NMNAT/WLD(S)-mediated axon protection independent of axonal mitochondria.
Kitay BM, McCormack R, Wang Y, Tsoulfas P, Zhai RG. Kitay BM, et al. Hum Mol Genet. 2013 Apr 15;22(8):1601-14. doi: 10.1093/hmg/ddt009. Epub 2013 Jan 11. Hum Mol Genet. 2013. PMID: 23314018 Free PMC article. - NAD+ Metabolism, Metabolic Stress, and Infection.
Groth B, Venkatakrishnan P, Lin SJ. Groth B, et al. Front Mol Biosci. 2021 May 19;8:686412. doi: 10.3389/fmolb.2021.686412. eCollection 2021. Front Mol Biosci. 2021. PMID: 34095234 Free PMC article. Review. - NAD+ metabolism: pathophysiologic mechanisms and therapeutic potential.
Xie N, Zhang L, Gao W, Huang C, Huber PE, Zhou X, Li C, Shen G, Zou B. Xie N, et al. Signal Transduct Target Ther. 2020 Oct 7;5(1):227. doi: 10.1038/s41392-020-00311-7. Signal Transduct Target Ther. 2020. PMID: 33028824 Free PMC article. Review. - Wld S requires Nmnat1 enzymatic activity and N16-VCP interactions to suppress Wallerian degeneration.
Avery MA, Sheehan AE, Kerr KS, Wang J, Freeman MR. Avery MA, et al. J Cell Biol. 2009 Feb 23;184(4):501-13. doi: 10.1083/jcb.200808042. J Cell Biol. 2009. PMID: 19237597 Free PMC article. - Alternative splicing of Drosophila Nmnat functions as a switch to enhance neuroprotection under stress.
Ruan K, Zhu Y, Li C, Brazill JM, Zhai RG. Ruan K, et al. Nat Commun. 2015 Nov 30;6:10057. doi: 10.1038/ncomms10057. Nat Commun. 2015. PMID: 26616331 Free PMC article.
References
- MacDonald JM, et al. The Drosophila cell corpse engulfment receptor draper mediates glial clearance of severed axons. Neuron. 2006;50:869–881. - PubMed
- Mack TG, et al. Wallerian degeneration of injured axons and synapses is delayed by a Ube4b/Nmnat chimeric gene. Nature Neurosci. 2001;4:1199–1206. - PubMed
- Ribchester RR, et al. Persistence of neuromuscular junctions after axotomy in mice with slow Wallerian degeneration (C57BL/WldS) Eur J Neurosci. 1995;7:1641–1650. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous