Foxn4 directly regulates tbx2b expression and atrioventricular canal formation - PubMed (original) (raw)
Foxn4 directly regulates tbx2b expression and atrioventricular canal formation
Neil C Chi et al. Genes Dev. 2008.
Abstract
Cardiac chamber formation represents an essential evolutionary milestone that allows for the heart to receive (atrium) and pump (ventricle) blood throughout a closed circulatory system. Here, we reveal a novel transcriptional pathway between foxn4 and tbx genes that facilitates this evolutionary event. We show that the zebrafish gene slipjig, which encodes Foxn4, regulates the formation of the atrioventricular (AV) canal to divide the heart. sli/foxn4 is expressed in the AV canal, and its encoded product binds to a highly conserved tbx2 enhancer domain that contains Foxn4- and T-box-binding sites, both necessary to regulate tbx2b expression in the AV canal.
Figures
Figure 1.
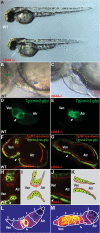
Failure of cardiac looping, malformation of AV canal, and loss of AV conduction delay in slipjig mutants. (A) Bright-field micrographs of 48-hpf wild-type (WT) and sli mutant (_s644_−/−) embryos. The white arrow points to pericardial edema. (B,C) Nomarski micrographs of wild-type and sli mutant hearts at 48 hpf. (B) Green circle delineates the wild-type ventricle, and blue circle indicates the wild-type atrium. (C) Yellow circle delineates the sli mutant heart with no discernible AV boundary. (D,E) Fluorescence micrographs of 48-hpf Tg(cmlc2:gfp) wild-type and sli mutant hearts. sli mutant hearts fail to loop and to form an AV canal. (F–H,J) Confocal micrographs of 40-hpf Tg(cmlc2:ras-GFP)s883; Tg(flk1:ras-cherry)s896 wild-type (F) and sli mutant (G) hearts and AV canal region (H,J). Myocardium and endocardium are in green and red, respectively. sli mutant AV myocardial and endocardial cells fail to undergo characteristic cell shape changes. (I,K) Schematic representation of wild-type and sli mutant AV region. (L,M) Forty-hour-post-fertilzation Tg(cmlc2:gCaMP)s878 wild-type and sli mutant heart optical maps of calcium-dependent fluorescence represented by isochronal lines every 60 msec. Numbers indicate temporal sequence of calcium activation in the heart. sli mutant hearts fail to develop an AV conduction delay by 40 hpf. The white arrowheads point to the AV canal. (Atr) Atrium; (Ven) ventricle.
Figure 2.
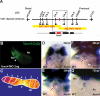
Molecular analyses of AV-specific cardiac genes reveal AV canal defects in sli mutant hearts. Schematized representations are shown to the right of whole-mount RNA in situ hybridization data. Red lines indicate myocardium, green lines indicate endocardium, and blue lines indicate gene expression. In 48-hpf wild-type hearts, bmp4 (A,B), tbx2b (E,F), and versican (I,J) are expressed in the AV myocardium and notch1b (M,N) is expressed in the AV endocardium. In 48-hpf sli mutant hearts, bmp4 (C,D) and versican (K,L) expression is expanded throughout the ventricular myocardium, tbx2b (G,H) expression is absent within the AV canal, and notch1b (O,P) expression is diffusely expanded throughout the endocardium. The red arrowhead points to the AV canal. The red box surrounds sli mutant hearts. (A) Atrium; (V) ventricle.
Figure 3.
sli encodes Foxn4. (A) Genetic map of the sli region. Numbers below SSLP markers indicate recombination events. Two genes were identified within the critical region, which spans two BACs. (B) Knockdown of foxn4 by the injection of 2 ng of an ATG MO into Tg(cmlc2:GFP) embryos results in failure of the hearts to form an AV canal constriction (arrowhead) and absence of cardiac looping. (C) Calcium-dependent fluorescence optical map of 48-hpf Tg(cmlc2:gCaMP)s878 foxn4 MO knockdown heart reveals loss of AV conduction delay at 48 hpf. Isochronal lines depict the distance traveled every 60 msec. Numbers indicate temporal sequence of calcium activation in the heart. (D–G) foxn4 is expressed in the AV canal at 24, 36, 48, and 72 hpf. The black arrows point to the AV canal. The white dashed lines outline the heart. (D) Dorsal view. (E–G) Ventral view. (Atr) atrium; (Ven) ventricle.
Figure 4.
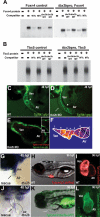
Foxn4 and Tbx5 directly regulate expression of tbx2b, a gene required for AV canal formation. (A,B) EMSA reveals specific binding of Foxn4 and Tbx5 to their respective binding elements within the tbx2b enhancer. Lane 1 (−/−) contains reticulocyte lysate without recombinant Foxn4 or Tbx5 protein. Recombinant Foxn4 (A) and Tbx5 (B) proteins were combined with radiolabeled double-stranded oligonucleotides representing the canonical Foxn4-binding site (Foxn4 control) and Tbx5-binding site (Tbx5 control) as well as the tbx2b enhancer Foxn4-binding element (_tbx2b_pro, Foxn4) and Tbx5-binding element (_tbx2b_pro, Tbx5). Foxn4 and Tbx5 interactions to respective binding sites were further tested through competition assays using excess unlabeled canonical wild-type/mutant (WTc, MTc) and tbx2b enhancer wild-type/mutant (tbx2b WT, tbx2b MT), foxn4, and tbx5 oligonucleotides. (C–F) tbx2b MO knockdown hearts exhibit absence of AV canal and cardiac looping at 48 hpf. Fluorescence micrographs of 48-hpf control (C) and tbx2b MO-injected (D) Tg(flk1:gfp)843 embryos. The arrowheads point to the AV boundary. (E) Confocal micrograph of 48-hpf Tg(cmlc2:ras-GFP)s883; Tg(flk1:ras-cherry)s896 tbx2b MO knockdown heart. (Inset) AV boundary region. (F) Calcium-dependent fluorescence optical map of 48-hpf Tg(cmlc2:gCaMP)s878 tbx2b MO knockdown heart reveals loss of AV conduction delay at 48 hpf. Isochronal lines depict the distance traveled every 60 msec. Numbers indicate temporal sequence of calcium activation in the heart. (G,J) tbx2b expression in foxn4 mRNA rescued sli mutant heart. The black arrow points to the AV canal. (H,I,K,L) Cardiac-specific overexpression of tbx2b results in lack of cardiac looping and failure to form the AV canal. Fluorescence micrographs of 80-hpf Tg(cmlc2:dsRed)s879 wild-type (H,I) and Tg(cmlc2:Tbx2b-GFP)s900 (K,L) hearts. The white arrow points to pericardial edema.
Comment in
- A house with many rooms: how the heart got its chambers with foxn4.
Cohen ED, Morrisey EE. Cohen ED, et al. Genes Dev. 2008 Mar 15;22(6):706-10. doi: 10.1101/gad.1662408. Genes Dev. 2008. PMID: 18347088 Free PMC article. No abstract available.
Similar articles
- A house with many rooms: how the heart got its chambers with foxn4.
Cohen ED, Morrisey EE. Cohen ED, et al. Genes Dev. 2008 Mar 15;22(6):706-10. doi: 10.1101/gad.1662408. Genes Dev. 2008. PMID: 18347088 Free PMC article. No abstract available. - The mediator complex subunit Med10 regulates heart valve formation in zebrafish by controlling Tbx2b-mediated Has2 expression and cardiac jelly formation.
Just S, Hirth S, Berger IM, Fishman MC, Rottbauer W. Just S, et al. Biochem Biophys Res Commun. 2016 Sep 2;477(4):581-588. doi: 10.1016/j.bbrc.2016.06.088. Epub 2016 Jun 22. Biochem Biophys Res Commun. 2016. PMID: 27343557 - Cooperative action of Tbx2 and Nkx2.5 inhibits ANF expression in the atrioventricular canal: implications for cardiac chamber formation.
Habets PE, Moorman AF, Clout DE, van Roon MA, Lingbeek M, van Lohuizen M, Campione M, Christoffels VM. Habets PE, et al. Genes Dev. 2002 May 15;16(10):1234-46. doi: 10.1101/gad.222902. Genes Dev. 2002. PMID: 12023302 Free PMC article. - Tbx20, Smads, and the atrioventricular canal.
Singh R, Kispert A. Singh R, et al. Trends Cardiovasc Med. 2010 May;20(4):109-14. doi: 10.1016/j.tcm.2010.09.004. Trends Cardiovasc Med. 2010. PMID: 21335279 Review. - Foxn4: a multi-faceted transcriptional regulator of cell fates in vertebrate development.
Xiang M, Li S. Xiang M, et al. Sci China Life Sci. 2013 Nov;56(11):985-93. doi: 10.1007/s11427-013-4543-8. Epub 2013 Sep 5. Sci China Life Sci. 2013. PMID: 24008385 Review.
Cited by
- Hemodynamics regulate spatiotemporal artery muscularization in the developing circle of Willis.
Cheng S, Xia IF, Wanner R, Abello J, Stratman AN, Nicoli S. Cheng S, et al. bioRxiv [Preprint]. 2024 Apr 12:2023.12.01.569622. doi: 10.1101/2023.12.01.569622. bioRxiv. 2024. PMID: 38077062 Free PMC article. Updated. Preprint. - Loss of ALS-associated TDP-43 in zebrafish causes muscle degeneration, vascular dysfunction, and reduced motor neuron axon outgrowth.
Schmid B, Hruscha A, Hogl S, Banzhaf-Strathmann J, Strecker K, van der Zee J, Teucke M, Eimer S, Hegermann J, Kittelmann M, Kremmer E, Cruts M, Solchenberger B, Hasenkamp L, van Bebber F, Van Broeckhoven C, Edbauer D, Lichtenthaler SF, Haass C. Schmid B, et al. Proc Natl Acad Sci U S A. 2013 Mar 26;110(13):4986-91. doi: 10.1073/pnas.1218311110. Epub 2013 Mar 1. Proc Natl Acad Sci U S A. 2013. PMID: 23457265 Free PMC article. - Defining hepatic dysfunction parameters in two models of fatty liver disease in zebrafish larvae.
Howarth DL, Yin C, Yeh K, Sadler KC. Howarth DL, et al. Zebrafish. 2013 Jun;10(2):199-210. doi: 10.1089/zeb.2012.0821. Epub 2013 May 22. Zebrafish. 2013. PMID: 23697887 Free PMC article. - Sema3d controls collective endothelial cell migration by distinct mechanisms via Nrp1 and PlxnD1.
Hamm MJ, Kirchmaier BC, Herzog W. Hamm MJ, et al. J Cell Biol. 2016 Nov 7;215(3):415-430. doi: 10.1083/jcb.201603100. Epub 2016 Oct 31. J Cell Biol. 2016. PMID: 27799363 Free PMC article. - Two developmentally distinct populations of neural crest cells contribute to the zebrafish heart.
Cavanaugh AM, Huang J, Chen JN. Cavanaugh AM, et al. Dev Biol. 2015 Aug 15;404(2):103-12. doi: 10.1016/j.ydbio.2015.06.002. Epub 2015 Jun 15. Dev Biol. 2015. PMID: 26086691 Free PMC article.
References
- Basson C.T., Bachinsky D.R., Lin R.C., Levi T., Elkins J.A., Soults J., Grayzel D., Kroumpouzou E., Traill T.A., Leblanc-Straceski J., et al. Mutations in human TBX5 cause limb and cardiac malformation in Holt-Oram syndrome. Nat. Genet. 1997;15:30–35. - PubMed
- Beis D., Bartman T., Jin S.W., Scott I.C., D’Amico L.A., Ober E.A., Verkade H., Frantsve J., Field H.A., Wehman A., et al. Genetic and cellular analyses of zebrafish atrioventricular cushion and valve development. Development. 2005;132:4193–4204. - PubMed
- Chen J.N., Haffter P., Odenthal J., Vogelsang E., Brand M., van Eeden F.J., Furutani-Seiki M., Granato M., Hammerschmidt M., Heisenberg C.P., et al. Mutations affecting the cardiovascular system and other internal organs in zebrafish. Development. 1996;123:293–302. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases