The insulator factor CTCF controls MHC class II gene expression and is required for the formation of long-distance chromatin interactions - PubMed (original) (raw)
The insulator factor CTCF controls MHC class II gene expression and is required for the formation of long-distance chromatin interactions
Parimal Majumder et al. J Exp Med. 2008.
Abstract
Knockdown of the insulator factor CCCTC binding factor (CTCF), which binds XL9, an intergenic element located between HLA-DRB1 and HLA-DQA1, was found to diminish expression of these genes. The mechanism involved interactions between CTCF and class II transactivator (CIITA), the master regulator of major histocompatibility complex class II (MHC-II) gene expression, and the formation of long-distance chromatin loops between XL9 and the proximal promoter regions of these MHC-II genes. The interactions were inducible and dependent on the activity of CIITA, regulatory factor X, and CTCF. RNA fluorescence in situ hybridizations show that both genes can be expressed simultaneously from the same chromosome. Collectively, the results suggest a model whereby both HLA-DRB1 and HLA-DQA1 loci can interact simultaneously with XL9, and describe a new regulatory mechanism for these MHC-II genes involving the alteration of the general chromatin conformation of the region and their regulation by CTCF.
Figures
Figure 1.
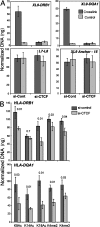
CTCF knockdown reduces HLA-DRB1 and HLA-DQA1 mRNA levels. (A) An overall schematic of the HLA-DRB1 and HLA-DQA1 gene and XL9 region is shown. The conserved proximal promoter elements of the MHC-II genes, W-X-Y, where RFX and CIITA interact are indicated. (B) siRNA to CTCF but not an irrelevant siRNA knocked down the expression of CTCF protein. Raji cells were transiently transfected with SMART pool siRNAs to CTCF or GFP and assayed by Western blotting for CTCF, RFX5, and β-actin expression at the indicated time points. (C) CTCF siRNA–transfected Raji cells displayed a marked decrease in HLA-DRB1 and HLA-DQA1 mRNA levels as determined by real-time RT-PCR. The decrease in expression of these genes was specific, as a nonspecific siRNA had no effect and CIITA and RFX5 gene expression were not significantly altered. This analysis was performed three times. The mean of each experiment (with SEM) is shown with the data normalized to the levels of GAPDH. GAPDH mRNA levels were unaltered during the course of this analysis.
Figure 2.
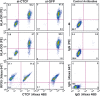
CTCF knockdown results in a loss of surface HLA-DR and HLA-DQ expression. Raji cells transiently transfected for 72 h with siRNA to CTCF or to GFP as in Fig. 1 were stained for intracellular CTCF and surface MHC-II expression. Two populations of cells exist for the CTCF siRNA panels, identifying those cells that were efficiently transfected from those that were not. GFP siRNA had no effect on the levels of MHC-II or CTCF. siRNA-transfected cells were also stained for CTCF, followed by RFX5 staining. No change in RFX5 levels were observed. The fluorophores used for detection are indicated (PE and Alexa Fluor 488). Control staining patterns for intracellular and surface antibodies are shown. This figure is representative of two independent experiments.
Figure 3.
CIITA associates with CTCF. Cellular lysates from Raji (wild-type), RJ2.2.5 (CIITA−, RFX5+), RJ-CIITA (CIITA+, RFX5+), and SJO (CIITA+, RFX5−) cells were prepared and immunoprecipitated with the indicated antibodies. The immunoprecipitates were analyzed by Western blotting for the presence of (A) CIITA, (B, top) CTCF, and (B, bottom) RFX5. CIITA was found in immunoprecipitates using CTCF, and CTCF was found in coimmunoprecipitates using CIITA and RFX5 antisera in Raji cells, indicating an association between the factors. The association was not observed in RJ2.2.5 cells with either CIITA or RFX5 antisera, suggesting that the associations between RFX5 and CTCF were dependent on the presence of CIITA. As in earlier papers (references 24, 25), RFX5 and CIITA interactions were also observed. No interactions with the nonspecific antibody to TCR were observed. 10% of the input is indicated (I). (C) Coimmunoprecipitation experiments using either CIITA or TCR antisera in the presence of ethidium bromide or treated with DNase I were performed as described in Materials and methods. The precipitations were analyzed by immunoblotting for RFX5 and CTCF as indicated. Each panel in this figure set is representative of at least two independent experiments.
Figure 4.
Long-range chromatin interactions form between XL9 and the proximal promoter regions of the HLA-DRB1 and HLA-DQA1 genes. (A) A schematic of the HLA-DRB1 and HLA-DQA1 gene region depicts the locations of the _Eco_RI sites (top tick marks) and the primers used (bottom tick marks) in the 3C assays. The position of XL9 is indicated by a thick purple vertical bar. In the 3C assay, chromatin isolated from formaldehyde–cross-linked cells was digested overnight with a large excess of _Eco_RI. After inactivation of the enzyme, samples were diluted and T4 DNA ligase was added. Novel ligation junctions were detected by PCR using the primer sets indicated and 35 cycles of amplification. 3C PCR primers P-1 with P-2 and P-3 with P-4 (red) allow detection of the XL9 restriction fragment with HLA-DRB1 and HLA-DQA1, respectively. (B) Interactions between XL9 and HLA-DRB1 or HLA-DQA1 are specific and dependent on the presence of CIITA and RFX5. A 3C product was observed in Raji cells when formaldehyde (CH2O) cross-linking and T4 DNA ligase were included during the assay. Intact chromatin was necessary for the formation of the 3C product, as no product was observed with purified genomic DNA. 3C product formation required CIITA and RFX5, as no products were observed for RJ2.2.5 or SJO cells, which are deficient for CIITA and RFX5, respectively. A 3C product was observed in RJ-CIITA cells, which are RJ2.2.5 cells stably complemented with CIITA. PCR assays using nonspecific control primers NC-5 (A, green) with P-1 or NC-6 (A, green) with P-3 were used to demonstrate that random ligation of their encoding restriction fragments did not occur with HLA-DRB1 and HLA-DQA1, respectively. Loading control primers L-7 and L-8 (blue) amplify DNA contained within a single _Eco_RI fragment and serve as a loading control. Fivefold less DNA was added to the PCR reaction for the loading controls. (C) The 3C assay was performed on freshly isolated CD19+ human peripheral B lymphocytes. The experimental conditions were identical to those described in B, with two concentrations (50 and 100 ng) of genomic DNA used for the PCR step. gen, genomic DNA.
Figure 5.
XL9 interacts with HLA-DRB1 and HLA-DQA1. To ascertain if XL9 interacted with other restriction fragments within the HLA-DRB1, HLA-DQA1 region, a quantitative 3C assay was used across the region. (A) A schematic showing the subregion and each _Eco_RI site numbered 1–23 is shown. The orientation of primers specific to these sites is indicated by black arrowheads. XL9 primers, which served as “anchors” for each 3C PCR, are shown by a single red arrowhead. Because of the differences in optimal annealing temperature between the restriction fragment primers, multiple XL9 anchor primers were required to produce single PCR products with similar efficiencies. Ovals represent the respective W-X-Y box regions. Primers 22 and 24 (blue arrowheads) were used to detect interactions between HLA-DRB1 and HLA-DQA1, as described in C. (B) 3C assays were performed on Raji and RJ2.2.5 cells as in Fig. 4. Each 3C assay was compared with its own standard curve generated from the amplification of an _HLA-DRB1_– and _HLA-DQA1_–containing BAC that had been _Eco_RI digested and ligated. The results from three separate experiments were averaged and normalized against the BAC DNA used in the standard curve. The results are plotted with the standard deviation observed. Primer set 15 with its XL9 anchor serves as a loading control for the system, as this ligation product is derived from a single _Eco_RI fragment (XL9). Note that primers 6 and 20 represent the same _Eco_RI fragments as primers NC-5 and NC-6 from Fig. 4. (C) Raji and RJ2.2.5 cells were used to examine whether the HLA-DRB1 and HLA-DQA1 promoter regions interact. A 3C product between HLA-DRB1 (primer 24) and HLA-DQA1 (primer 22) promoter-containing restriction fragments was detected that was dependent on the presence of CIITA. Student's t tests were used to determine the significance of differences between Raji and RJ2.2.5 samples for each primer set. In B, only 3C samples using XL9 anchors with primers 3 and 22 showed significance (P < 0.04). The differences in C were found to be significant (P < 0.03).
Figure 6.
IFN-γ induces long-range interactions between XL9 and the proximal promoter regions of HLA-DRB1 and HLA-DQA1. MHC-II–negative A431 epithelial cells were treated with IFN-γ in a time course that extended to 24 h. (A) mRNA analysis, (B) Western blots, and (C) 3C analysis were conducted at the indicated time points. (A) Real-time RT-PCR was performed with _CIITA_-, _HLA-DRB1_–, and _HLA-DQA1_–specific primers on RNA isolated at the indicated time points after IFN-γ treatment. The crossover threshold (Ct) real-time PCR values were normalized to the Ct values of those obtained for GAPDH mRNA and presented as the mean fold induction with standard error. (B) A Western blot shows that CIITA protein can be detected as early as 6 h after IFN-γ induction and that the expression of CIITA is maintained over the 24-h time course. (C) The 3C assays were performed on the A431 cells as in Fig. 4. Interactions between XL9 and the HLA-DQA1 and HLA-DRB1 promoter regions were observed at 12 h after the induction time point. All assays were performed at least three times from independently treated cultures.
Figure 7.
CTCF is required for interactions between XL9 and the HLA-DRB1 and HLA-DQA1 promoter regions. 72 h after CTCF siRNA transfection, Raji cells were labeled with anti–HLA-DR magnetic beads and separated from nontransfected cells by MACS column separation. The HLA-DR–negative cells were assayed by 3C and compared with siRNA GFP–transfected cells. Cells were either cross-linked or left untreated (control) before 3C. Quantitative 3C primer sets to detect XL9/HLA-DRB1 and XL9/HLA-DQA1, as indicated in the figure, were used as described in Fig. 5 and Fig. S5. Loading control primer sets from Fig. 4 (L-7/L-8) and 3C ligation control primers (15/XL9 anchor) from Fig. 5 were also used. 3C products were quantitated as in Fig. 5. Data from three independent chromatin preparations were averaged and plotted with their standard deviation. (B) ChIP assays for histone modifications at the HLA-DRB1 and HLA-DQA1 promoter regions were conducted using the indicated antibodies 72 h after cells were transfected with either control or CTCF siRNAs. The real-time PCR data were normalized to input and to a standard curve for each locus, and the mean of three independent assays is shown with standard deviation. Student's t test p-values are shown above each comparison.
Figure 8.
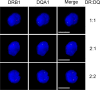
Transcription of HLA-DRB1 and HLA-DQA1 genes. RNA FISH for HLA-DRB1 and HLA-DQA1 mRNA in Raji cells displayed a variety of expression patterns, suggesting that the neighboring genes could be coexpressed. Three HLA-DRB1 and HLA-DQA1 coexpression patterns are shown. The complete analysis of all expression patterns is shown in Table I. PCR probes corresponding to the middle of each of these genes, including intronic sequences, were used. Cells are counterstained with DAPI to highlight the nucleus. No expression was observed in RJ2.2.5 cells (not depicted). Bars, 10 μM.
Figure 9.
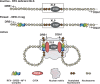
Models of interactions between HLA-DRB1 and HLA-DQA1 and the XL9 region. Three potential states are shown. The inactive state represents RFX-deficient cell types as in SJO cells from bare lymphocyte syndrome patients with no factors bound to the MHC-II proximal regulatory regions. In the inactive state, no interactions with XL9 occur and the proximal regulatory regions of MHC-II genes display background chromatin marks associated with the gene activation. A poised state is suggested for normal MHC-II–nonexpressing cells. In such cells, RFX–CREB–NF-Y but not CIITA are assembled at the MHC-II proximal regulatory regions. No interactions with XL9 occur in the poised state, but chromatin marks associated with genes that can be activated are present. In MHC-II–expressing cells, CIITA is expressed and an “active state” is proposed with the proximal promoter regions of the HLA-DRB1 and/or HLA-DQA1 genes interacting directly with XL9. The potential for both genes to interact with XL9 is depicted, but it is possible that only one gene interacts or is transcribed at a time.
Comment in
- The role of CTCF in regulating nuclear organization.
Williams A, Flavell RA. Williams A, et al. J Exp Med. 2008 Apr 14;205(4):747-50. doi: 10.1084/jem.20080066. Epub 2008 Mar 17. J Exp Med. 2008. PMID: 18347103 Free PMC article.
Similar articles
- Regulation of major histocompatibility complex class II genes.
Choi NM, Majumder P, Boss JM. Choi NM, et al. Curr Opin Immunol. 2011 Feb;23(1):81-7. doi: 10.1016/j.coi.2010.09.007. Epub 2010 Oct 21. Curr Opin Immunol. 2011. PMID: 20970972 Free PMC article. Review. - The human major histocompatibility complex class II HLA-DRB1 and HLA-DQA1 genes are separated by a CTCF-binding enhancer-blocking element.
Majumder P, Gomez JA, Boss JM. Majumder P, et al. J Biol Chem. 2006 Jul 7;281(27):18435-43. doi: 10.1074/jbc.M601298200. Epub 2006 May 4. J Biol Chem. 2006. PMID: 16675454 - Impact of human sepsis on CCCTC-binding factor associated monocyte transcriptional response of Major Histocompatibility Complex II components.
Siegler BH, Uhle F, Lichtenstern C, Arens C, Bartkuhn M, Weigand MA, Weiterer S. Siegler BH, et al. PLoS One. 2018 Sep 13;13(9):e0204168. doi: 10.1371/journal.pone.0204168. eCollection 2018. PLoS One. 2018. PMID: 30212590 Free PMC article. - CTCF controls expression and chromatin architecture of the human major histocompatibility complex class II locus.
Majumder P, Boss JM. Majumder P, et al. Mol Cell Biol. 2010 Sep;30(17):4211-23. doi: 10.1128/MCB.00327-10. Epub 2010 Jun 28. Mol Cell Biol. 2010. PMID: 20584980 Free PMC article. - Roles of chromatin insulator proteins in higher-order chromatin organization and transcription regulation.
Vogelmann J, Valeri A, Guillou E, Cuvier O, Nollmann M. Vogelmann J, et al. Nucleus. 2011 Sep-Oct;2(5):358-69. doi: 10.4161/nucl.2.5.17860. Epub 2011 Sep 1. Nucleus. 2011. PMID: 21983085 Free PMC article. Review.
Cited by
- Interchromosomal association and gene regulation in trans.
Williams A, Spilianakis CG, Flavell RA. Williams A, et al. Trends Genet. 2010 Apr;26(4):188-97. doi: 10.1016/j.tig.2010.01.007. Epub 2010 Mar 16. Trends Genet. 2010. PMID: 20236724 Free PMC article. Review. - Mechanisms of cohesin-mediated gene regulation and lessons learned from cohesinopathies.
Ball AR Jr, Chen YY, Yokomori K. Ball AR Jr, et al. Biochim Biophys Acta. 2014 Mar;1839(3):191-202. doi: 10.1016/j.bbagrm.2013.11.002. Epub 2013 Nov 22. Biochim Biophys Acta. 2014. PMID: 24269489 Free PMC article. Review. - DNA methylation dysregulates and silences the HLA-DQ locus by altering chromatin architecture.
Majumder P, Boss JM. Majumder P, et al. Genes Immun. 2011 Jun;12(4):291-9. doi: 10.1038/gene.2010.77. Epub 2011 Feb 17. Genes Immun. 2011. PMID: 21326318 Free PMC article. - PML promotes MHC class II gene expression by stabilizing the class II transactivator.
Ulbricht T, Alzrigat M, Horch A, Reuter N, von Mikecz A, Steimle V, Schmitt E, Krämer OH, Stamminger T, Hemmerich P. Ulbricht T, et al. J Cell Biol. 2012 Oct 1;199(1):49-63. doi: 10.1083/jcb.201112015. Epub 2012 Sep 24. J Cell Biol. 2012. PMID: 23007646 Free PMC article. - Regulation of major histocompatibility complex class II genes.
Choi NM, Majumder P, Boss JM. Choi NM, et al. Curr Opin Immunol. 2011 Feb;23(1):81-7. doi: 10.1016/j.coi.2010.09.007. Epub 2010 Oct 21. Curr Opin Immunol. 2011. PMID: 20970972 Free PMC article. Review.
References
- Germain, R.N., N.S. Braunstein, M.A. Brown, L.H. Glimcher, R.I. Lechler, J. McCluskey, D.H. Margulies, J. Miller, M.A. Norcross, W.E. Paul, et al. 1986. Structure and function of murine class II major histocompatibility complex genes. Mt. Sinai J. Med. 53:194–201. - PubMed
- Collins, T., A.J. Korman, C.T. Wake, J.M. Boss, D.J. Kappes, W. Fiers, K.A. Ault, M.A.J. Gimbrone, J.L. Strominger, and J.S. Pober. 1984. Immune interferon activates multiple class II major histocompatibility complex genes and the associated invariant chain gene in human endothelial cells and dermal fibroblasts. Proc. Natl. Acad. Sci. USA. 81:4917–4921. - PMC - PubMed
- Reith, W., and B. Mach. 2001. The bare lymphocyte syndrome and the regulation of MHC expression. Annu. Rev. Immunol. 19:331–373. - PubMed
- Ting, J.P., and J. Trowsdale. 2002. Genetic control of MHC class II expression. Cell. 109(Suppl.):S21–S33. - PubMed
- Boss, J.M., and P.E. Jensen. 2003. Transcriptional regulation of the MHC class II antigen presentation pathway. Curr. Opin. Immunol. 15:105–111. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM047310-14/GM/NIGMS NIH HHS/United States
- R01 GM047310/GM/NIGMS NIH HHS/United States
- GM47310/GM/NIGMS NIH HHS/United States
- R01 GM073120/GM/NIGMS NIH HHS/United States
- GM073120/GM/NIGMS NIH HHS/United States
- T32 GM008490/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials