The Th17-ELR+ CXC chemokine pathway is essential for the development of central nervous system autoimmune disease - PubMed (original) (raw)
The Th17-ELR+ CXC chemokine pathway is essential for the development of central nervous system autoimmune disease
Thaddeus Carlson et al. J Exp Med. 2008.
Abstract
The ELR(+) CXC chemokines CXCL1 and CXCL2 are up-regulated in the central nervous system (CNS) during multiple sclerosis (MS) and its animal model, experimental autoimmune encephalomyelitis (EAE). However, their functional significance and the pathways regulating their expression are largely unknown. We show that transfer of encephalitogenic CD4(+) Th17 cells is sufficient to induce CXCL1 and CXCL2 transcription in the spinal cords of naive, syngeneic recipients. Blockade or genetic silencing of CXCR2, a major receptor for these chemokines in mice, abrogates blood-brain barrier (BBB) breakdown, CNS infiltration by leukocytes, and the development of clinical deficits during the presentation as well as relapses of EAE. Depletion of circulating polymorphonuclear leukocytes (PMN) had a similar therapeutic effect. Furthermore, injection of CXCR2(+) PMN into CXCR2(-/-) mice was sufficient to restore susceptibility to EAE. Our findings reveal that a Th17-ELR(+) CXC chemokine pathway is critical for granulocyte mobilization, BBB compromise, and the clinical manifestation of autoimmune demyelination in myelin peptide-sensitized mice, and suggest new therapeutic targets for diseases such as MS.
Figures
Figure 1.
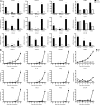
ELR+ CXC chemokines are up-regulated in the CNS during preclinical and active stages of EAE. (A) SJL mice were immunized with PLP139-151 or NP260-283 (control) in CFA. Spinal cord RNA was isolated from PLP139-151–primed mice during distinct stages of EAE, or from NP260-283–primed mice at analogous time points after immunization, for real-time RT-PCR analysis. Data represent fold induction compared with naive spinal cords (n = 4 mice per group). (B) RNA was extracted from spinal cords between days 8 and 12 after immunization with PLP139-151 or NP260-283 for analysis by real-time RT-PCR (n = 5 mice per group). *, P < 0.05 for PLP-immunized mice compared with NP-immunized mice. n.s., not significantly different from naive spinal cords.
Figure 2.
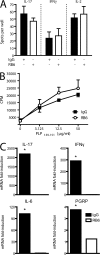
Transfer of myelin-reactive Th17 cells induces CNS expression of CXCL1 and CXCL2. LN cells from donor SJL mice immunized with PLP139-151 in IFA were cultured with antigen in the presence of either recombinant IL-23 (for the generation of Th17 cells), IL-12 (for the generation of Th1 cells), or anti–IL-12/23 p40 neutralizing antibody (for the generation of lineage-uncommitted cells). (A, left and middle) Supernatants were collected serially and subjected to ELISA for measurement of IL-17 and IFN-γ levels. (A, right) Some wells were pulsed with tritiated thymidine at 72 h and harvested 12 h later for measurement of radioisotope incorporation. Data represent mean ± SD. (B) Spinal cords were harvested from hosts that had been injected with either Th17, Th1, or uncommitted PLP139-151–reactive T cells 10 d earlier (n = 5 mice per group). RNA was extracted for analysis by real-time RT-PCR. The results show mean relative expression of the specified transcripts in cords from host mice to cords from naive mice. A representative experiment of four is shown. *, P < 0.05 compared with naive mice.
Figure 3.
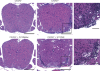
CXCR2 plays a critical role in the presentation and relapse of EAE. Mice immunized with PLP139-151 in CFA were treated with anti-CXCR2 antiserum or NRS between days 8 and 14 (A and C–E) or days 18 and 24 (B) after immunization. (A and B) Mice were rated for clinical signs of EAE on a daily basis. (C) BBB integrity was assessed on day 14 after immunization by Evans blue dye extravasation. Relative permeability is calculated as follows: (μg Evans blue/g spinal cord)/( μg Evans blue/g kidney). Data represent mean ± SD. *, P < 0.05 compared with naive mice. (D) Spinal cords were fixed on day 14 after immunization. Representative hematoxylin and eosin sections are shown from five mice per group. Bars: (left and right) 200 μm; (middle) 50 μm. (E) Spinal cords were sampled at the time of peak disease in the group injected with NRS and analyzed by real-time RT-PCR. Data represent fold induction relative to naive spinal cords (NRS, n = 4; anti-CXCR2, n = 3). *, P < 0.05 for anti-CXCR2– compared with NRS-treated mice. (F) CD4+ T cells, purified from draining lymph nodes on day 14 after immunization, were stimulated with naive T cell–depleted splenocytes and 25 μg/ml PLP139-151 in vitro. Lymphoproliferation was measured by uptake of [3H]thymidine. Data represent mean ± SD. (G) BALB/c CXCR2+/+, CXCR2+/−, and CXCR2−/− mice were immunized with PLP185-206 in CFA to induce EAE. The mean daily clinical score of each group is shown (+/+, n = 5; +/−, n = 8; −/−, n = 7). The experiment was repeated five times with similar results.
Figure 4.
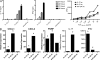
PMN accumulate in the blood and infiltrate the CNS during preclinical and active phases of EAE. (A and B) PBLs were analyzed by flow cytometry to determine the percentage of PMN (identified as Ly6G+, 7/4+, MHC class II− cells). (A) Representative FACS profiles. (B) Percentages of PMN among PBLs were averaged over four mice per group. *, P < 0.001 compared with naive mice. (C and D) Spinal cord–infiltrating cells were isolated and pooled from PLP139-151–immunized SJL mice (n = 10–20) immediately before, or on the day of, clinical EAE onset. Cells from mice immunized with NP260-183 served as controls. PMN were identified by FACS analysis as in A. (C) Representative FACS profiles gated on MHC class II− cells. (D) Total number of cells per cord and the percentage of CNS-infiltrating PMN. Data represent mean ± SD of four independent experiments. *, P < 0.05 compared with control. (E) Histological sections of spinal cords from PLP139-151– and NP260-283–immunized mice were Giemsa stained. PMN (arrows) are identified by their characteristic nuclear morphology (insets). The sections shown are representative of four mice per group. All experiments were repeated three or more times with consistent results. Bars, 50 μm.
Figure 5.
PMN depletion prevents BBB disruption and clinical and histopathological manifestations of EAE. SJL mice were injected with either RB6 or control IgG (0.5 mg per dose) every other day from days 8–16 (A–C, F, and G) or days 21–27 (D and E) after immunization with PLP139-151 in CFA. (A) PBLs were collected at serial time points and analyzed by flow cytometry. PMN were identified as CD11b+, 7/4+, MHC class II− cells. The percentage of PMN was averaged over six mice per group. A representative FACS profile of each group is shown. Data represent mean ± SD. (B) Daily clinical scores were averaged over six mice in each group. (C) BBB integrity was assessed during the indicated stages of EAE in the control IgG–treated group by Evans blue dye extravasation. Data represent mean ± SD of six mice per group. *, P < 0.05 compared with naive mice. (D) The mean daily clinical score of each group (n = 8) is shown. (E) BBB integrity was assessed during the time of relapse in the control IgG–treated group. Data represent mean ± SD of six mice per group. *, P < 0.05 compared with naive mice. (F and G) Spinal cords were harvested and fixed on day 14 after immunization with PLP139-151. Sections were Giemsa stained to visualize cell morphology. Images are representative of sections from four mice per group. All experiments were repeated three or more times with consistent results. Bars: (F, left; and G) 200 μm; (F, right) 50 μm.
Figure 6.
PLP-specific peripheral CD4+ responses are not altered by PMN depletion, but CNS up-regulation of inflammatory markers is blocked. (A and B) CD4+ T cells were isolated from draining lymph nodes of PLP-immunized SJL mice that had been treated with either control IgG (closed bars and squares) or RB6 (open bars and circles). The purified T cells were cultured with naive T cell–depleted splenocytes with or without 25 μg/ml PLP139151 for ELISPOT (A) and [3H]thymidine uptake proliferation (B) assays. The ELISPOT data shown were generated by subtracting background spots (10 or fewer) that appeared in the absence of antigenic challenge. Data represent mean ± SD. (C) Spinal cords from PLP-immunized mice that had been treated with either control IgG or RB6 were analyzed by real-time RT-PCR. Data represent fold induction relative to naive spinal cords (n = 5 mice per group). *, P < 0.05 for IgG- compared with RB6-treated mice.
Figure 7.
WT PMN promote neuroinflammation in myelin-immunized CXCR2−/− mice. BALB/c CXCR2+/+ and CXCR2−/− mice were immunized with PLP185-206 in CFA. CXCR2−/− mice were injected with 5 × 106 purified bone marrow PMN or BMMac daily between days 10 and 14 after immunization. Spinal cords were removed between days 13 and 15 after immunization, fixed, and hematoxylin and eosin stained. Representative sections of three mice per group are shown. Bars: (left and middle) 200 μm; (right) 50 μm.
Figure 8.
WT PMN induce BBB breakdown and CNS cytokine expression in myelin-immunized CXCR2−/− mice but do not affect peripheral CD4+ T cell responses. BALB/c CXCR2+/+ and CXCR2−/− mice were immunized with PLP185-206 in CFA. CXCR2−/− mice were injected with 5 × 106 purified bone marrow PMN or BMMac daily between days 10 and 14 after immunization. (A) Cerebrovascular permeability was assessed by Evans blue dye extravasation on day 14 after immunization. Data represent mean ± SD. *, P < 0.05 compared with naive mice. (B) RNA was isolated from spinal cords between days 13 and 15 after immunization and analyzed by real-time RT-PCR. Data represent fold induction compared with naive spinal cords (n = 4 mice per group). *, P < 0.03 compared with CXCR2−/− mice. n.d., not detectable. (C and D) CD4+ T cells were isolated from draining lymph nodes on day 14 after immunization. The purified T cells were cultured with naive T cell–depleted splenocytes plus or minus PLP139-151. Lymphoproliferative and cytokine responses were measured by [3H]thymidine uptake (C) and ELISPOT (D) assays, respectively. The ELISPOT data shown were generated by subtracting background spots (six or fewer) that appeared in the absence of antigenic challenge. Data represent mean ± SD.
Similar articles
- A protective role for ELR+ chemokines during acute viral encephalomyelitis.
Hosking MP, Liu L, Ransohoff RM, Lane TE. Hosking MP, et al. PLoS Pathog. 2009 Nov;5(11):e1000648. doi: 10.1371/journal.ppat.1000648. Epub 2009 Nov 6. PLoS Pathog. 2009. PMID: 19893623 Free PMC article. - An IFNγ/CXCL2 regulatory pathway determines lesion localization during EAE.
Stoolman JS, Duncker PC, Huber AK, Giles DA, Washnock-Schmid JM, Soulika AM, Segal BM. Stoolman JS, et al. J Neuroinflammation. 2018 Jul 16;15(1):208. doi: 10.1186/s12974-018-1237-y. J Neuroinflammation. 2018. PMID: 30012158 Free PMC article. - Neutrophil-related factors as biomarkers in EAE and MS.
Rumble JM, Huber AK, Krishnamoorthy G, Srinivasan A, Giles DA, Zhang X, Wang L, Segal BM. Rumble JM, et al. J Exp Med. 2015 Jan 12;212(1):23-35. doi: 10.1084/jem.20141015. Epub 2015 Jan 5. J Exp Med. 2015. PMID: 25559893 Free PMC article. - The C-C Chemokines CCL17 and CCL22 and Their Receptor CCR4 in CNS Autoimmunity.
Scheu S, Ali S, Ruland C, Arolt V, Alferink J. Scheu S, et al. Int J Mol Sci. 2017 Nov 2;18(11):2306. doi: 10.3390/ijms18112306. Int J Mol Sci. 2017. PMID: 29099057 Free PMC article. Review. - Role of chemokines in CNS health and pathology: a focus on the CCL2/CCR2 and CXCL8/CXCR2 networks.
Semple BD, Kossmann T, Morganti-Kossmann MC. Semple BD, et al. J Cereb Blood Flow Metab. 2010 Mar;30(3):459-73. doi: 10.1038/jcbfm.2009.240. Epub 2009 Nov 11. J Cereb Blood Flow Metab. 2010. PMID: 19904283 Free PMC article. Review.
Cited by
- The Role of the Intestinal Microbiome in Multiple Sclerosis-Lessons to Be Learned from Hippocrates.
El-Sayed MM, Mohak S, Gala D, Fabian R, Peterfi Z, Fabian Z. El-Sayed MM, et al. Biology (Basel). 2023 Nov 24;12(12):1463. doi: 10.3390/biology12121463. Biology (Basel). 2023. PMID: 38132289 Free PMC article. Review. - The chemokine receptor CXCR2 and coronavirus-induced neurologic disease.
Weinger JG, Marro BS, Hosking MP, Lane TE. Weinger JG, et al. Virology. 2013 Jan 5;435(1):110-7. doi: 10.1016/j.virol.2012.08.049. Virology. 2013. PMID: 23217621 Free PMC article. Review. - Discovery of CNS Penetrant CXCR2 Antagonists for the Potential Treatment of CNS Demyelinating Disorders.
Xu H, Lu H, Xu Z, Luan L, Li C, Xu Y, Dong K, Zhang J, Li X, Li Y, Liu G, Gong S, Zhao YG, Liu A, Zhang Y, Zhang W, Cai X, Xiang JN, Elliott JD, Lin X. Xu H, et al. ACS Med Chem Lett. 2016 Feb 8;7(4):397-402. doi: 10.1021/acsmedchemlett.5b00489. eCollection 2016 Apr 14. ACS Med Chem Lett. 2016. PMID: 27096048 Free PMC article. - GM-CSF-dependent, CD103+ dermal dendritic cells play a critical role in Th effector cell differentiation after subcutaneous immunization.
King IL, Kroenke MA, Segal BM. King IL, et al. J Exp Med. 2010 May 10;207(5):953-61. doi: 10.1084/jem.20091844. Epub 2010 Apr 26. J Exp Med. 2010. PMID: 20421390 Free PMC article. - Effect of CXCR2 Inhibition on Behavioral Outcomes and Pathology in Rat Model of Neuromyelitis Optica.
Jones MV, Levy M. Jones MV, et al. J Immunol Res. 2018 Dec 13;2018:9034695. doi: 10.1155/2018/9034695. eCollection 2018. J Immunol Res. 2018. PMID: 30648122 Free PMC article.
References
- Fischer, H.G., and G. Reichmann. 2001. Brain dendritic cells and macrophages/microglia in central nervous system inflammation. J. Immunol. 166:2717–2726. - PubMed
- Traugott, U., C.S. Raine, and D.E. McFarlin. 1985. Acute experimental allergic encephalomyelitis in the mouse: immunopathology of the developing lesion. Cell. Immunol. 91:240–254. - PubMed
- Karpus, W.J., and R.M. Ransohoff. 1998. Chemokine regulation of experimental autoimmune encephalomyelitis: temporal and spatial expression patterns govern disease pathogenesis. J. Immunol. 161:2667–2671. - PubMed
- Rebenko-Moll, N.M., L. Liu, A. Cardona, and R.M. Ransohoff. 2006. Chemokines, mononuclear cells and the nervous system: heaven (or hell) is in the details. Curr. Opin. Immunol. 18:683–689. - PubMed
- Fischer, F.R., L. Santambrogio, Y. Luo, M.A. Berman, W.W. Hancock, and M.E. Dorf. 2000. Modulation of experimental autoimmune encephalomyelitis: effect of altered peptide ligand on chemokine and chemokine receptor expression. J. Neuroimmunol. 110:195–208. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS041249/NS/NINDS NIH HHS/United States
- R01 NS047687/NS/NINDS NIH HHS/United States
- NS047687-01A1/NS/NINDS NIH HHS/United States
- NS041249/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials