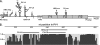A morpholino oligomer targeting highly conserved internal ribosome entry site sequence is able to inhibit multiple species of picornavirus - PubMed (original) (raw)
A morpholino oligomer targeting highly conserved internal ribosome entry site sequence is able to inhibit multiple species of picornavirus
Jeffrey K Stone et al. Antimicrob Agents Chemother. 2008 Jun.
Abstract
Members of the genera Enterovirus and Rhinovirus (family Picornaviridae) cause a wide range of human diseases. An established vaccine is available only for poliovirus, and no effective therapy is available for the treatment of infections caused by any pathogenic picornavirus. Peptide-conjugated phosphorodiamidate morpholino oligomers (PPMO) are single-stranded DNA-like antisense agents that readily enter cells. A panel of PPMO was tested for their antiviral activities against various picornaviruses. PPMO targeting conserved internal ribosome entry site (IRES) sequence were highly active against human rhinovirus type 14, coxsackievirus type B2, and poliovirus type 1 (PV1), reducing PV1 titers by up to 6 log(10) in cell cultures. Comparative sequence analysis led us to design a PPMO (EnteroX) targeting 22 nucleotides of IRES sequence that are perfectly conserved across greater than 99% of all human enteroviruses and rhinoviruses. EnteroX reduced PV1 replication in cell culture to an extent similar to that of other IRES-specific PPMO. Resistant PV1 arose in cell cultures after 12 passages in the presence of EnteroX and were found to have two mutations within the EnteroX target sequence. Nevertheless, cPVR transgenic mice treated once daily by intraperitoneal (i.p.) injection with EnteroX before and/or after i.p. infection with 3 x 10(8) PFU (three times the 50% lethal dose) of PV1 had an approximately 80% higher rate of survival than the controls. The viral titer in tissues taken at day 5 postinfection showed that animals in the EnteroX-treated group averaged over 3, 4, and 5 log(10) less virus in the small intestine, spinal cord, and brain, respectively, than the amount in the control animals. These results suggest that EnteroX may have broad therapeutic potential against entero- and rhinoviruses.
Figures
FIG. 1.
PPMO target sites in viral RNA and sequence conservation in the stem-loop 5 region of human entero- and rhinoviruses. (A) Schematic of a composite entero- and rhinovirus genome. The VPg protein is indicated by a black dot at the 5′ end of the genome; and the names of other features of the viral genome are in italicized font, including the cloverleaf region (′CL') and the other five stem-loop regions of the 5′ UTR (denoted with roman numerals), the initiator AUG, and the poly(A) tail. The bracketed region before the AUG represents the ∼100-nt difference in length between the HRV and PV1 5′ UTRs. PPMO target sites are in nonitalicized font and are indicated by thin lines and arrows. (B) Histogram showing sequence conservation in the stem-loop 5 region of human entero- and rhinoviruses. A total of 196 full-length genomic sequences were aligned and analyzed (see Materials and Methods). An arbitrary scale is depicted at the left of the histogram, where 1.0 denotes perfect agreement at a nucleotide position across all entries. The relative target sites of the two versions of EnteroX and the PV-L5 PPMO are shown above the histogram.
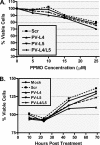
FIG. 2.
Evaluation of PPMO cytotoxicity in cell cultures. (A) HeLa cell viability was measured by the MTS assay after 24 h of treatment with the indicated concentrations of the indicated PPMO. (B) HeLa cells were incubated with 10 μM of the indicated PPMO, and the cell viability then measured at the indicated times after the initiation of treatment. The percentage of viable cells was obtained by comparison to the number of mock (water)-treated cells, which was set equal to 100%. Each treatment in both of the experiments is the average for 12 wells.
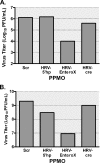
FIG. 3.
EnteroX PPMO inhibits HRV14 and CVB2 in cell cultures. HeLa cells were treated with 5 μM of the indicated PPMO after a 1-h period of infection with HRV14 at an MOI of 0.03 (A) or a clinical isolate of CVB2 at an MOI of 0.05 (B). Viral titers were determined from samples taken at 24 h p.i. (CVB2) or 48 h p.i. (HRV14) by plaque assay. In both of the experiments, mock (water)-treated cells produced titers that were almost identical to those produced by Scr PPMO-treated cells. The average of two experiments is shown.
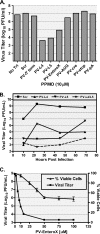
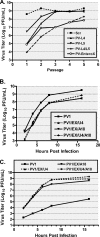
FIG. 4.
PPMO targeting the IRES sequence inhibit PV1, and PPMO can be used in combination to more effectively limit PV1 infections of cell cultures. HeLa cells were incubated with 10 μM of the indicated PPMO before and after infection with PV1 (MOI, 0.1), and the viral titers at 10 h p.i. (A) or the indicated time points p.i. (B) were determined by plaque assay. In each of the experiments, the average of three experiments is shown. (C) HeLa cells were treated with the indicated concentrations of PV-EnteroX before and after infection with PV1 (MOI, 0.1). Viral titers were determined by the plaque assay at 21 h p.i. (▪). As well, the viability of noninfected HeLa cells was measured by the MTS assay 21 h after the initiation of treatment with the indicated concentrations of PV-EnteroX (▴). The average for triplicate samples is shown.
FIG. 5.
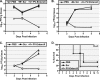
PPMO targeting the IRES and AUG region of viral reporter replicon RNA restrict translation in a potent and specific manner. (A) Schematic of the PV1-Luc replicon pRib(+)RLuc. (B) In-cell translation assay. Cells treated with indicated PPMO at 10 μM were transfected with PV1-Luc RNA [produced from pRib(+)RLuc] and then treated with GuaHCl (Gua), an inhibitor of viral replication. The cells were then assayed for their Luc levels at the indicated times. The translation of PV1-Luc RNA was severely limited by PPMO that specifically target the IRES, as measured by a decrease in the total reflective light units (RLU). The level of suppression produced by antisense PPMO was similar to that observed with puromycin, a known inhibitor of translation. (C) PPMO failed to inhibit the translation of capped Luc RNA [produced from pRib(+)T7Luc] under the same conditions described above for panel B, indicating that PPMO inhibition of translation is sequence specific. (D) Cell-free Luc assay. HRV14-Luc RNA transcripts (produced from HRV14/ΔP1Luc; see Materials and Methods) and the indicated PPMO were mixed in rabbit reticulocyte lysate translation reaction mixtures. The Luc activity is charted in comparison to that from the mock-treated control reactions. PPMO targeting the IRES and AUG region effectively suppressed the translation of HRV-Luc RNA, while PPMO targeting HRV elements thought to have a regulatory role in HRV RNA synthesis did not suppress translation. The average of two experiments is shown in each graph.
FIG. 6.
PV1 can escape the antiviral effects of PPMO in cell cultures through target-specific mutations. (A) PV1 (MOI, 0.1) was sequentially passaged in HeLa cells in the presence of 5 to 10 μM PPMO until complete CPE was observed. Virus was then isolated and used to initiate the next round of infection. After 12 passages, PV-EnteroX-resistant PV1 was isolated and sequenced. Two nucleotide mutations were found in the PV-EnteroX target region, and these were then cloned either singly or doubly into pRib(+)XpA. Mutant clones (see Materials and Methods for the nomenclature) were used to generate mutant viral RNA, which was transfected into HeLa cells treated with 10 μM PV-EnteroX (EX) and allowed to grow until CPE was observed. These P0 populations were then used at an MOI of 10 to infect HeLa cells that either had not (B) or had (C) been treated with 10 μM PV-EnteroX. Virus was taken at the indicated times (hours) p.i., and the titers determined by plaque assay. The viral titers shown are the averages of three experiments.
FIG. 7.
PV-EnteroX PPMO treatment limits viral titers in infected tissues and protects PV1-infected mice from death. Viral titers from the small intestines (A), spinal cords (B), and brain tissues (C) of cPVR mice receiving i.p. treatment with PBS or 200 μg (∼10 mg/kg) of the indicated PPMO at 48 and 24 h before, 1 h after, and then once a day on days 1 to 5 after intraperitoneal infection with 1 × 108 PFU of wt poliovirus (n = 6 per group per time point) are shown. (D) Survival plot of cPVR mice after treatment and infection, as described above (n = 18 per group).
Similar articles
- Inhibition of RNA virus infections with peptide-conjugated morpholino oligomers.
Stein DA. Stein DA. Curr Pharm Des. 2008;14(25):2619-34. doi: 10.2174/138161208786071290. Curr Pharm Des. 2008. PMID: 18991679 Review. - Inhibition of coxsackievirus B3 in cell cultures and in mice by peptide-conjugated morpholino oligomers targeting the internal ribosome entry site.
Yuan J, Stein DA, Lim T, Qiu D, Coughlin S, Liu Z, Wang Y, Blouch R, Moulton HM, Iversen PL, Yang D. Yuan J, et al. J Virol. 2006 Dec;80(23):11510-9. doi: 10.1128/JVI.00900-06. Epub 2006 Sep 20. J Virol. 2006. PMID: 16987987 Free PMC article. - In vitro resistance selection and in vivo efficacy of morpholino oligomers against West Nile virus.
Deas TS, Bennett CJ, Jones SA, Tilgner M, Ren P, Behr MJ, Stein DA, Iversen PL, Kramer LD, Bernard KA, Shi PY. Deas TS, et al. Antimicrob Agents Chemother. 2007 Jul;51(7):2470-82. doi: 10.1128/AAC.00069-07. Epub 2007 May 7. Antimicrob Agents Chemother. 2007. PMID: 17485503 Free PMC article. - Inhibition of enterovirus 71 infection by antisense octaguanidinium dendrimer-conjugated morpholino oligomers.
Tan CW, Chan YF, Quah YW, Poh CL. Tan CW, et al. Antiviral Res. 2014 Jul;107:35-41. doi: 10.1016/j.antiviral.2014.04.004. Epub 2014 Apr 24. Antiviral Res. 2014. PMID: 24769243 Free PMC article. - Antiviral agents against picornaviruses.
Eggers HJ. Eggers HJ. Antiviral Res. 1985;Suppl 1:57-65. doi: 10.1016/s0166-3542(85)80009-7. Antiviral Res. 1985. PMID: 3002267 Review. No abstract available.
Cited by
- Antisense Phosphorodiamidate Morpholino Oligomers as Novel Antiviral Compounds.
Nan Y, Zhang YJ. Nan Y, et al. Front Microbiol. 2018 Apr 20;9:750. doi: 10.3389/fmicb.2018.00750. eCollection 2018. Front Microbiol. 2018. PMID: 29731743 Free PMC article. Review. - Antisense phosphorodiamidate morpholino oligomers targeted to an essential gene inhibit Burkholderia cepacia complex.
Greenberg DE, Marshall-Batty KR, Brinster LR, Zarember KA, Shaw PA, Mellbye BL, Iversen PL, Holland SM, Geller BL. Greenberg DE, et al. J Infect Dis. 2010 Jun 15;201(12):1822-30. doi: 10.1086/652807. J Infect Dis. 2010. PMID: 20438352 Free PMC article. - The Interplay between Host Immunity and Respiratory Viral Infection in Asthma Exacerbation.
Hossain FMA, Choi JY, Uyangaa E, Park SO, Eo SK. Hossain FMA, et al. Immune Netw. 2019 Sep 9;19(5):e31. doi: 10.4110/in.2019.19.e31. eCollection 2019 Oct. Immune Netw. 2019. PMID: 31720042 Free PMC article. Review. - Enteroviruses: Classification, Diseases They Cause, and Approaches to Development of Antiviral Drugs.
Nikonov OS, Chernykh ES, Garber MB, Nikonova EY. Nikonov OS, et al. Biochemistry (Mosc). 2017 Dec;82(13):1615-1631. doi: 10.1134/S0006297917130041. Biochemistry (Mosc). 2017. PMID: 29523062 Free PMC article. Review. - Recent developments in antiviral agents against enterovirus 71 infection.
Tan CW, Lai JK, Sam IC, Chan YF. Tan CW, et al. J Biomed Sci. 2014 Feb 12;21(1):14. doi: 10.1186/1423-0127-21-14. J Biomed Sci. 2014. PMID: 24521134 Free PMC article. Review.
References
- Abes, S., H. M. Moulton, P. Clair, P. Prevot, D. S. Youngblood, R. P. Wu, P. L. Iversen, and B. Lebleu. 2006. Vectorization of morpholino oligomers by the (R-Ahx-R)4 peptide allows efficient splicing correction in the absence of endosomolytic agents. J. Control. Release 116:304-313. - PubMed
- Agol, V. I. 2002. Picornavirus genome: an overview, p. 127-148. In B. L. Semler and E. Wimmer (ed.), Molecular biology of picornaviruses. ASM Press, Washington, DC.
- Aylward, R. B. 2006. Eradicating polio: today's challenges and tomorrow's legacy. Ann. Trop. Med. Parasitol. 100:401-413. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources