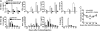HDAC inhibitor reduces cytokine storm and facilitates induction of chimerism that reverses lupus in anti-CD3 conditioning regimen - PubMed (original) (raw)
HDAC inhibitor reduces cytokine storm and facilitates induction of chimerism that reverses lupus in anti-CD3 conditioning regimen
Nainong Li et al. Proc Natl Acad Sci U S A. 2008.
Abstract
In allogeneic hematopoietic cell transplantation (HCT), donor T cell-mediated graft versus host leukemia (GVL) and graft versus autoimmune (GVA) activity play critical roles in treatment of hematological malignancies and refractory autoimmune diseases. However, graft versus host disease (GVHD), which sometimes can be fatal, remains a major obstacle in classical HCT, where recipients are conditioned with total body irradiation or high-dose chemotherapy. We previously reported that anti-CD3 conditioning allows donor CD8(+) T cells to facilitate engraftment and mediate GVL without causing GVHD. However, the clinical application of this radiation-free and GVHD preventative conditioning regimen is hindered by the cytokine storm syndrome triggered by anti-CD3 and the high-dose donor bone marrow (BM) cells required for induction of chimerism. Histone deacetylase (HDAC) inhibitors such as suberoylanilide hydroxamic acid (SAHA) are known to induce apoptosis of cancer cells and reduce production of proinflammatory cytokines by nonmalignant cells. Here, we report that SAHA inhibits the proliferative and cytotoxic activity of anti-CD3-activated T cells. Administration of low-dose SAHA reduces cytokine production and ameliorates the cytokine storm syndrome triggered by anti-CD3. Conditioning with anti-CD3 and SAHA allows induction of chimerism with lower doses of donor BM cells in old nonautoimmune and autoimmune lupus mice. In addition, conditioning with anti-CD3 and SAHA allows donor CD8(+) T cell-mediated GVA activity to reverse lupus glomerulonephritis without causing GVHD. These results indicate that conditioning with anti-CD3 and HDAC inhibitors represent a radiation-free and GVHD-preventative regimen with clinical application potential.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
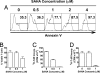
Fig. 1.
SAHA augmented apoptosis and inhibited proliferation of anti-CD3-activated T cells in a dose-dependent manner. (A) BALB/c spleen cells (0.5 × 106) were stimulated with plate-bound anti-CD3 in culture medium with titrated concentrations of SAHA (0 to 4 μM) for 72 h. Thereafter, cells were stained with anti-TCRαβ, DAPI, and annexin V. TCRαβ+ cells were gated and shown in a histogram of annexin V. The percentages of annexin V+ cells are shown for each culture. One representative FACS pattern of four replicated experiments is shown. The mean ± SE of the percentage of annexin V+ cells under different SAHA concentration (0 to 4 μM) were 38.1 ± 6.5%, 41.5 ± 7.2%, 64.4 ± 6.6%, 85.5 ± 2.7%, and 97.1 ± 1.5%. (B) Percentage of viable T cells among total T cells in the culture with SAHA at concentration of 0 to 1 μM. (C) T cell proliferation by anti-CD3 stimulation with SAHA at concentration of 0 to 1 μM. (D) T cell proliferation by allo-APC stimulation with SAHA at concentration of 0 to ≈1 μM. B–D show mean ± SE of four replicated experiments.
Fig. 2.
SAHA reduced in vitro and in vivo cytokine production triggered by anti-CD3. (A) BALB/c spleen cells (0.5 × 106) were stimulated with plate-bound anti-CD3 in the presence of SAHA at concentrations of 0, 0.5, and 1 μM. IL-2, IFN-γ, TNF-α, and IL-6 in culture supernatant were measured at 24, 48, and 72 h after culture. Mean ± SE of four replicated experiments are shown. (B) BALB/c mice were i.v. injected with anti-CD3 (5 μg/g) with or without coinjection of SAHA (40 μg/g) 12 and 1 h before anti-CD3 injection. Serum cytokines were measured kinetically (0.5 to ≈24 h) after anti-CD3 injection. Mean ± SE of four mice at each time point is shown. (C) Kinetic body temperature change of mice injected with anti-CD3 alone or anti-CD3 and SAHA. There were seven mice in each group.
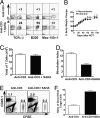
Fig. 3.
SAHA augmented induction of chimerism in old BALB/c mice. Old (>16 weeks) BALB/c mice were conditioned with anti-CD3 alone or anti-CD3 and SAHA and transplanted with C57BL/6 donor BM (2 × 106) and CD4+ T-depleted spleen cells (4 × 106). The recipients were monitored for clinical signs of GVHD daily and body weight weekly. (A) At 8 weeks after HCT, blood mononuclear cells were stained for H-2b (donor marker), TCRαβ, B220, and Mac-1/Gr-1. The percentages of donor T, B, and macrophage cells are shown. One representative sample of 12 mice in each group is shown. (B) Body weight change curves of HCT recipients conditioned with anti-CD3 alone (nonchimeric) or anti-CD3 plus SAHA (chimeric). There were 12 mice in each group. Mean ± SE is shown at each time point. (C) Yield of residual DAPI− live T cells in spleen of mice conditioned with anti-CD3 or anti-CD3 plus SAHA 9 days after anti-CD3 injection at the day of HCT. (D) Sorted T cells (0.2 × 106) from the spleen of conditioned BALB/c mice 9 days after anti-CD3 injection were stimulated with C57BL/6 dendritic cells (0.1 × 106) for 5 days. The proliferation was measured with 3H-TdR incorporation, and the stimulation index was calculated by formula: {[cpm of responder × stimulator] − [cpm of responder alone]} ÷ [cpm of responder alone]. Mean ± SE of four mice in each group is shown. (E) At 5 days after HCT, donor-type spleen cells from congenic C57BL/6 (CD45.1) and CFSE-labeled host-type spleen cells from BALB/c were injected into recipients conditioned with anti-CD3 alone or anti-CD3 plus SAHA. Then, 18 h later, spleen cells were harvested and stained with anti-CD45.1. Staining is shown in CD45.1 versus CFSE. One representative FACS pattern of four mice in each group is shown. The mean ± SE of CD45.1+ or CFSE+ cells in recipients conditioned with anti-CD3 alone versus recipients conditioned with anti-CD3 plus SAHA were 1.21 ± 0.07% versus 1.80 ± 0.08% or 0.35 ± 0.03% versus 0.20 ± 0.01%. (F) The ratio of residual CD45.1+ donor-type cells versus CFSE+ host-type cells were calculated, and mean ± SE of 4 recipients in each group is shown.
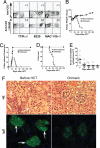
Fig. 4.
Conditioning with anti-CD3 and SAHA allowed induction of complete chimerism and reversed severe glomerulonephritis. Old NZB/W F1 mice (> 7 months) with severe proteinuria were conditioned with anti-CD3 and SAHA and transplanted with C57BL/6 donor BM (2 × 106/g) and CD4+ T-depleted spleen cells (4 × 106/g). The recipients were monitored for clinical signs of GVHD daily and body weight and proteinuria twice a week. The recipients were checked for chimerism 8 weeks after HCT. (A) Blood mononuclear cells of the anti-CD3 and SAHA-conditioned mice with or without HCT were stained for H-2b (donor marker) versus TCRαβ, B220, or Mac-1/Gr-1. The percentage of donor-type T, B, and macrophage cells is shown. One representative of 10 mice in each group is shown. (B) Body weight change curves of the mice given conditioning alone or conditioning and HCT over a 180-day period after HCT. Mean ± SE of 10 mice in each group is shown. (C and D), Proteinuria change curve and survival curve of the lupus mice given conditioning alone or conditioning and HCT. (E) Kinetic changes of serum levels of anti-dsDNA IgG2a antibodies in lupus mice given conditioning and HCT. (F) Hematoxylin/eosin staining of kidney tissues and immunofluorescent staining of IgG deposition in glomeruli of the lupus mice before treatment and 180 days after HCT. One representative sample of four examined mice in each group is shown.
Similar articles
- Donor CD8+ T cells facilitate induction of chimerism and tolerance without GVHD in autoimmune NOD mice conditioned with anti-CD3 mAb.
Liang Y, Huang T, Zhang C, Todorov I, Atkinson M, Kandeel F, Forman S, Zeng D. Liang Y, et al. Blood. 2005 Mar 1;105(5):2180-8. doi: 10.1182/blood-2004-06-2411. Epub 2004 Sep 16. Blood. 2005. PMID: 15374883 - Donor CD8+ T cells mediate graft-versus-leukemia activity without clinical signs of graft-versus-host disease in recipients conditioned with anti-CD3 monoclonal antibody.
Zhang C, Lou J, Li N, Todorov I, Lin CL, Cao YA, Contag CH, Kandeel F, Forman S, Zeng D. Zhang C, et al. J Immunol. 2007 Jan 15;178(2):838-50. doi: 10.4049/jimmunol.178.2.838. J Immunol. 2007. PMID: 17202345 - Protective conditioning against GVHD and graft rejection after combined organ and hematopoietic cell transplantation.
Strober S. Strober S. Blood Cells Mol Dis. 2008 Jan-Feb;40(1):48-54. doi: 10.1016/j.bcmd.2007.06.019. Epub 2007 Sep 10. Blood Cells Mol Dis. 2008. PMID: 17827036 Review.
Cited by
- Immunomodulatory effects of deacetylase inhibitors: therapeutic targeting of FOXP3+ regulatory T cells.
Wang L, de Zoeten EF, Greene MI, Hancock WW. Wang L, et al. Nat Rev Drug Discov. 2009 Dec;8(12):969-81. doi: 10.1038/nrd3031. Epub 2009 Oct 26. Nat Rev Drug Discov. 2009. PMID: 19855427 Free PMC article. Review. - Combining histone deacetylase inhibitor vorinostat with aurora kinase inhibitors enhances lymphoma cell killing with repression of c-Myc, hTERT, and microRNA levels.
Kretzner L, Scuto A, Dino PM, Kowolik CM, Wu J, Ventura P, Jove R, Forman SJ, Yen Y, Kirschbaum MH. Kretzner L, et al. Cancer Res. 2011 Jun 1;71(11):3912-20. doi: 10.1158/0008-5472.CAN-10-2259. Epub 2011 Apr 18. Cancer Res. 2011. PMID: 21502403 Free PMC article. - Advances in hematopoietic stem cell transplantation for autoimmune diseases.
Xu Y, Wang X, Hu Z, Huang R, Yang G, Wang R, Yang S, Guo L, Song Q, Wei J, Zhang X. Xu Y, et al. Heliyon. 2024 Oct 11;10(20):e39302. doi: 10.1016/j.heliyon.2024.e39302. eCollection 2024 Oct 30. Heliyon. 2024. PMID: 39492896 Free PMC article. Review. - A phase II study of vorinostat and rituximab for treatment of newly diagnosed and relapsed/refractory indolent non-Hodgkin lymphoma.
Chen R, Frankel P, Popplewell L, Siddiqi T, Ruel N, Rotter A, Thomas SH, Mott M, Nathwani N, Htut M, Nademanee A, Forman SJ, Kirschbaum M. Chen R, et al. Haematologica. 2015 Mar;100(3):357-62. doi: 10.3324/haematol.2014.117473. Epub 2015 Jan 16. Haematologica. 2015. PMID: 25596263 Free PMC article. Clinical Trial. - Treatment of HIV-Infected Individuals with the Histone Deacetylase Inhibitor Panobinostat Results in Increased Numbers of Regulatory T Cells and Limits Ex Vivo Lipopolysaccharide-Induced Inflammatory Responses.
Brinkmann CR, Højen JF, Rasmussen TA, Kjær AS, Olesen R, Denton PW, Østergaard L, Ouyang Z, Lichterfeld M, Yu X, Søgaard OS, Dinarello C, Tolstrup M. Brinkmann CR, et al. mSphere. 2018 Feb 14;3(1):e00616-17. doi: 10.1128/mSphere.00616-17. eCollection 2018 Jan-Feb. mSphere. 2018. PMID: 29468194 Free PMC article.
References
- Sullivan KM. In: Thomas' Hematopoietic Cell Transplantation. Blume KG, Forman SJ, Appelbaum FR, editors. Malden, MA: Blackwell; 2004. pp. 635–664.
- Sykes M, Nikolic B. Treatment of severe autoimmune disease by stem-cell transplantation. Nature. 2005;435:620–627. - PubMed
- Shizuru J. In: Thomas' Hematopoietic Cell Transplantation. Blume KG, Forman SJ, Appelbaum FR, editors. Malden, MA: Blackwell; 2004. pp. 324–343.
- Ferrara J, Antin J. In: Thomas' Hematopoietic Cell Transplantation. Blume KG, Forman SJ, Appelbaum FR, editors. Malden, MA: Blackwell; 2004. pp. 353–368.
- Shlomchik WD, et al. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 1999;285:412–415. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials