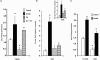Opposite regulation of CD36 ubiquitination by fatty acids and insulin: effects on fatty acid uptake - PubMed (original) (raw)
Opposite regulation of CD36 ubiquitination by fatty acids and insulin: effects on fatty acid uptake
Jill Smith et al. J Biol Chem. 2008.
Abstract
FAT/CD36 is a membrane scavenger receptor that facilitates long chain fatty acid uptake by muscle. Acute increases in membrane CD36 and fatty acid uptake have been reported in response to insulin and contraction. In this study we have explored protein ubiquitination as one potential mechanism for the regulation of CD36 level. CD36 expressed in Chinese hamster ovary (CHO) or HEK 293 cells was found to be polyubiquitinated via a process involving both lysines 48 and 63 of ubiquitin. Using CHO cells expressing the insulin receptor (CHO/hIR) and CD36, it is shown that addition of insulin (100 nm, 10 and 30 min) significantly reduced CD36 ubiquitination. In contrast, ubiquitination was strongly enhanced by fatty acids (200 microm palmitate or oleate, 2 h). Similarly, endogenous CD36 in C2C12 myotubes was ubiquitinated, and this was enhanced by oleic acid treatment, which also reduced total CD36 protein in cell lysates. Insulin reduced CD36 ubiquitination, increased CD36 protein, and inhibited the opposite effects of fatty acids on both parameters. These changes were paralleled by changes in fatty acid uptake, which could be blocked by the CD36 inhibitor sulfosuccinimidyl oleate. Mutation of the two lysine residues in the carboxyl-terminal tail of CD36 markedly attenuated ubiquitination of the protein expressed in CHO cells and was associated with increased CD36 level and enhanced oleate uptake and incorporation into triglycerides. In conclusion, fatty acids and insulin induce opposite alterations in CD36 ubiquitination, modulating CD36 level and fatty acid uptake. Altered CD36 turnover may contribute to abnormal fatty acid uptake in the insulin-resistant muscle.
Figures
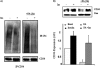
FIGURE 1.
Ubiquitination of CD36 in CHO/hIR cells. A, CHO/hIR cells were co-transfected with GFP-CD36 and FLAG-ubiquitin wild type (WT) or mutants K48R, K64R, or K/R (double mutant). The cells were lysed, and clarified lysates were immunoprecipitated (IP) with rabbit anti-GFP antibody. The immunocomplexes were resolved by SDS-PAGE and immunoblotted (IB) with antibody against FLAG. B, CHO/hIR cells were co-transfected with GFP-CD36 and FLAG-ubiquitin WT. The cell pellets were collected and boiled in PBS containing 1% SDS. The lysates were diluted 10-fold with lysis buffer prior to immunoprecipitation (IP) and Western blot analysis as described under A. The data shown are representative of two experiments.
FIGURE 2.
Ubiquitination of exogenous CD36 in CHO/hIR cells is modulated by insulin and FA. CHO/hIR cells were transfected with GFP alone or GFP-CD36. A, cells were starved and treated with 100 n
m
insulin (Ins) for the indicated times. The cells were lysed, and clarified lysates were immunoprecipitated (IP) with rabbit anti-GFP antibody. The immunocomplexes were resolved by SDS-PAGE and immunoblotted (IB) with antibodies recognizing ubiquitin (Ubi) or GFP (GFP alone does not appear on the gel because the molecular weight of unconjugated GFP is 28kD versus >110kDa for GFP-CD36). B, cells were treated with 200 μ
m
oleic acid complexed to BSA (FA:BSA = 1) for 2 h, immunoprecipitated (IP) and immunoblotted (IB) as described for A. Similar results were obtained with palmitic acid (data not shown). The data shown are representative of three experiments.
FIGURE 3.
Ubiquitination of endogenous CD36 in C2C12 myotubes and effects of insulin and FA. A, C2C12 myotubes were treated with 100 n
m
insulin (Ins) for 30 min and/or 200 μ
m
oleic acid (FA:BSA = 1) for 2 h as indicated. The cells were lysed, and clarified lysates were immunoprecipitated (IP) with rabbit anti-CD36 antibody. The immunocomplexes were resolved by SDS-PAGE and immunoblotted (IB) with ubiquitin antibody. B, whole cell lysates were analyzed for CD36 expression by Western blot (upper panel), Ran is the loading control. Signals were quantified by densitometry (lower panel) and CD36 level in FA-, insulin-, and FA- and insulin-treated cells was related to expression in untreated (basal) cells. Although a representative Western blot is shown, the densitometry data are the averages from three experiments with their standard errors. All of the treatments were significantly different (p < 0.05) from untreated cells.
FIGURE 4.
Effects of insulin and FA treatments on FA uptake and incorporation into triglycerides by C2C12 myotubes. C2C12 myotubes were treated with 100 n
m
insulin (Ins) for 30 min and/or 200 μ
m
oleic acid (FA: BSA = 1) for 2 h as indicated. The controls are untreated cells. A, fatty acid uptake values were derived from time courses (1–4 min) performed at room temperature. The data represent the means ± S.E. from six independent experiments. B, FA incorporation into TAG was measured as described under “Experimental Procedures.” The data represent the means ± S.E. from six experiments. Inset, the FA incorporation data after correction for dilution of the isotope by intracellular free FA measured by mass spectrometry. C, cells were treated with or without SSO prior to fatty acid uptake measurement. The data are the means ± S.E. from two experiments with quadruplicate. *, p < 0.05.
FIGURE 5.
Effects of MG132 on CD36 ubiquitination, CD36 protein levels and fatty acid uptake. A, HEK 293 cells were transfected with FLAG-CD36 construct. The cells were treated with MG132 (10 μ
m
) for the indicated times. The cells were lysed, and clarified lysates were immunoprecipitated (IP) with FLAG affinity gel. The immunocomplexes were eluted with FLAG peptide, resolved by SDS-PAGE, and immunoblotted (IB) with ubiquitin (Ubi) antibody. B, effect of MG132 (4 h) on CD36 ubiquitination in C2C12 myotubes. C, MG132 (4 h) treatment on FA-induced reduction in CD36 levels. The myotubes were treated with 200 μ
m
oleic acid (FA:BSA = 1) for 2 h with or without preincubation with MG132 (10 μ
m
) for 4 h. Whole cell lysates (WCL) were prepared and analyzed by immunoblotting. The data (A–C) are representative of two experiments with similar results. D, effect of SSO on FA uptake by myotubes. C2C12 myotubes were pretreated with 10 μ
m
MG132 for 4 h and/or SSO for 30 min as indicated. Fatty acid uptake rates are from time courses (1–4 min) conducted in quadruplicate from two independent experiments. The data are the means ± S.E. E, effect of MG132 on FA incorporation into TAG. The data (means ± S.E.) are from three independent experiments. *, p < 0.05.
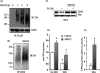
FIGURE 6.
CD36 is ubiquitinated at lysines 469 and 472. A, HEK 293 cells were transfected with FLAG-CD36 wild type (WT) or mutated CD36 with alanine substitutions at lysines 469 and 472, K469A/K472A mutant (K/A). The cells were lysed, and clarified lysates were immunoprecipitated (IP) with FLAG affinity gel. The immunocomplexes were eluted with FLAG peptide, resolved by SDS-PAGE, and immunoblotted (IB) with ubiquitin (Ubi) antibody. B, Cells transfected with WT or K/A FLAG-CD36 were labeled with biotin, and the amounts of CD36 protein on the cell surface and in total cell lysates were evaluated by Western blot and quantified by densitometry. WCL, whole cell lysates.
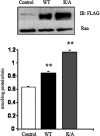
FIGURE 7.
Effects of the double CD36 lysine mutant on FA uptake in CHO/hIR cells. CHO/hIR cells were transfected with either FLAG-CD36 wild type (WT) or with CD36 mutated at both lysines 469 and 472 (K/A). The upper panel shows levels of expressed CD36 with Ran as a loading control (Ctrl). The lower panel shows fatty acid uptake rates (means ± S.E.) from two experiments conducted in quadruplicate. **, p < 0.01. IB, immunoblot.
Similar articles
- Fatty acid translocase (FAT/CD36) is localized on insulin-containing granules in human pancreatic beta-cells and mediates fatty acid effects on insulin secretion.
Noushmehr H, D'Amico E, Farilla L, Hui H, Wawrowsky KA, Mlynarski W, Doria A, Abumrad NA, Perfetti R. Noushmehr H, et al. Diabetes. 2005 Feb;54(2):472-81. doi: 10.2337/diabetes.54.2.472. Diabetes. 2005. PMID: 15677505 - Facilitation of fatty acid uptake by CD36 in insulin-producing cells reduces fatty-acid-induced insulin secretion and glucose regulation of fatty acid oxidation.
Wallin T, Ma Z, Ogata H, Jørgensen IH, Iezzi M, Wang H, Wollheim CB, Björklund A. Wallin T, et al. Biochim Biophys Acta. 2010 Feb;1801(2):191-7. doi: 10.1016/j.bbalip.2009.11.002. Epub 2009 Nov 18. Biochim Biophys Acta. 2010. PMID: 19931418 - Sulfo-N-succinimidyl oleate (SSO) inhibits fatty acid uptake and signaling for intracellular calcium via binding CD36 lysine 164: SSO also inhibits oxidized low density lipoprotein uptake by macrophages.
Kuda O, Pietka TA, Demianova Z, Kudova E, Cvacka J, Kopecky J, Abumrad NA. Kuda O, et al. J Biol Chem. 2013 May 31;288(22):15547-55. doi: 10.1074/jbc.M113.473298. Epub 2013 Apr 18. J Biol Chem. 2013. PMID: 23603908 Free PMC article. - Evidence for concerted action of FAT/CD36 and FABPpm to increase fatty acid transport across the plasma membrane.
Chabowski A, Górski J, Luiken JJ, Glatz JF, Bonen A. Chabowski A, et al. Prostaglandins Leukot Essent Fatty Acids. 2007 Nov-Dec;77(5-6):345-53. doi: 10.1016/j.plefa.2007.10.017. Prostaglandins Leukot Essent Fatty Acids. 2007. PMID: 18240411 Review. - Role of CD36 in membrane transport of long-chain fatty acids.
Ibrahimi A, Abumrad NA. Ibrahimi A, et al. Curr Opin Clin Nutr Metab Care. 2002 Mar;5(2):139-45. doi: 10.1097/00075197-200203000-00004. Curr Opin Clin Nutr Metab Care. 2002. PMID: 11844979 Review.
Cited by
- Cardiac fatty acid uptake and metabolism in the rat model of polycystic ovary syndrome.
Tepavčević S, Milutinović DV, Macut D, Stojiljković M, Nikolić M, Božić-Antić I, Ćulafić T, Bjekić-Macut J, Matić G, Korićanac G. Tepavčević S, et al. Endocrine. 2015 Sep;50(1):193-201. doi: 10.1007/s12020-015-0558-1. Epub 2015 Feb 22. Endocrine. 2015. PMID: 25702158 - The changes of cardiac energy metabolism with sodium-glucose transporter 2 inhibitor therapy.
Su S, Ji X, Li T, Teng Y, Wang B, Han X, Zhao M. Su S, et al. Front Cardiovasc Med. 2023 Dec 6;10:1291450. doi: 10.3389/fcvm.2023.1291450. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 38124893 Free PMC article. Review. - CD36 tango in cancer: signaling pathways and functions.
Wang J, Li Y. Wang J, et al. Theranostics. 2019 Jul 9;9(17):4893-4908. doi: 10.7150/thno.36037. eCollection 2019. Theranostics. 2019. PMID: 31410189 Free PMC article. Review. - Cellular fatty acid uptake: a pathway under construction.
Su X, Abumrad NA. Su X, et al. Trends Endocrinol Metab. 2009 Mar;20(2):72-7. doi: 10.1016/j.tem.2008.11.001. Epub 2009 Jan 29. Trends Endocrinol Metab. 2009. PMID: 19185504 Free PMC article. Review. - Inhibition of USP14 suppresses the formation of foam cell by promoting CD36 degradation.
Zhang F, Xia X, Chai R, Xu R, Xu Q, Liu M, Chen X, Liu B, Liu S, Liu N. Zhang F, et al. J Cell Mol Med. 2020 Mar;24(6):3292-3302. doi: 10.1111/jcmm.15002. Epub 2020 Jan 22. J Cell Mol Med. 2020. PMID: 31970862 Free PMC article.
References
- Hajri, T., and Abumrad, N. A. (2002) Annu. Rev. Nutr. 22 383–415 - PubMed
- Glatz, J. F., Luiken, J. J., and Bonen, A. (2001) J. Mol. Neurosci. 16 123–132 - PubMed
- Coburn, C. T., Knapp, F. F., Jr., Febbraio, M., Beets, A. L., Silverstein, R. L., and Abumrad, N. A. (2000) J. Biol. Chem. 275 32523–32529 - PubMed
- Harmon, C. M., and Abumrad, N. A. (1993) J. Membr. Biol. 133 43–49 - PubMed
- Ibrahimi, A., Bonen, A., Blinn, W. D., Hajri, T., Li, X., Zhong, K., Cameron, R., and Abumrad, N. A. (1999) J. Biol. Chem. 274 26761–26766 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 HL-007275/HL/NHLBI NIH HHS/United States
- P30 DK056341-08/DK/NIDDK NIH HHS/United States
- R01DK 33301/DK/NIDDK NIH HHS/United States
- DK56351/DK/NIDDK NIH HHS/United States
- 2R01GM42259/GM/NIGMS NIH HHS/United States
- P30 DK056341/DK/NIDDK NIH HHS/United States
- P30 DK056341-07/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous