Persistence of transmitted drug resistance among subjects with primary human immunodeficiency virus infection - PubMed (original) (raw)
Persistence of transmitted drug resistance among subjects with primary human immunodeficiency virus infection
Susan J Little et al. J Virol. 2008 Jun.
Abstract
Following interruption of antiretroviral therapy among individuals with acquired drug resistance, preexisting drug-sensitive virus emerges relatively rapidly. In contrast, wild-type virus is not archived in individuals infected with drug-resistant human immunodeficiency virus (HIV) and thus cannot emerge rapidly in the absence of selective drug pressure. Fourteen recently HIV-infected patients with transmitted drug-resistant virus were followed for a median of 2.1 years after the estimated date of infection (EDI) without receiving antiretroviral therapy. HIV drug resistance and pol replication capacity (RC) in longitudinal plasma samples were assayed. Resistance mutations were characterized as pure populations or mixtures. The mean time to first detection of a mixture of wild-type and drug-resistant viruses was 96 weeks (1.8 years) (95% confidence interval, 48 to 192 weeks) after the EDI. The median time to loss of detectable drug resistance using population-based assays ranged from 4.1 years (conservative estimate) to longer than the lifetime of the individual (less conservative estimate). The transmission of drug-resistant virus was not associated with virus with reduced RC. Sexual transmission of HIV selects for highly fit drug-resistant variants that persist for years. The prolonged persistence of transmitted drug resistance strongly supports the routine use of HIV resistance genotyping for all newly diagnosed individuals.
Figures
FIG. 1.
(a) Kaplan-Meier plot of the time to first detection of the WT virus as a mixture (WT/DR) in the population. (b) Kaplan-Meier plot of the time to complete replacement (i.e., last detection of any DR virus). Dashed lines indicate upper and lower 95% CIs.
FIG. 2.
Longitudinal FC susceptibility results for a representative drug for each subject. Red circles indicate EFV susceptibility. Blue circles indicate lopinavir susceptibility. Subject 01-0629, infected with a K103K/N mixture at baseline, did not show reduced EFV susceptibility at baseline or during follow-up. Subject 01-0180 showed a waning of reduced EFV susceptibility over time.
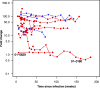
FIG. 3.
Estimation of the relative fitness advantage of WT virus over virus harboring K103N in patient 01-0180. (a) FC in susceptibility to EFV over time (points) and the fit of a model that assumes a log-linear relationship between FC and frequency of the WT variant (see Materials and Methods). (b) Relationship between the time during which a mixture persists and the selective advantage of WT.
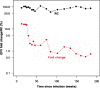
FIG. 4.
Dynamics of RC and FC in susceptibility to EFV in patient 01-0180. Despite a dramatic drop in FC, the RC remains relatively stable over the period of observation.
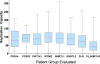
FIG. 5.
Box plots showing the median RC among eight different groups, with 25th and 75th percentiles shown by the box and the complete range indicated by whiskers. PDR14, study patients with transmitted DR virus; PDR33, 33 primary infection patients infected with DR virus but not included in present study due to antiretroviral treatment choices after entry; PWT141, 141 primary patients infected with WT virus; WT962, unrelated WT samples with infection of undetermined duration (n = 962); NNRTI-1, samples with an isolated single NNRTI major drug resistance mutation (n = 1,522); NNRTI-2, samples with only two major NNRTI drug resistance mutation (n = 236); PI-R, samples with ≥1 major PI mutation (no NRTI or NNRTI mutations) (n = 459); and PI_NRTI-R, samples with ≥1 major PI mutation and ≥1 major NRTI mutation (without NNRTI mutations).
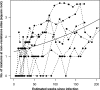
FIG. 6.
The total number of nonsynonymous mutations in predicted CTL epitopes between the baseline sequence and subsequent sequences was determined for all study participants over time. Overall, there is a large variation in the number of substitutions in each individual subject, though the mean number (represented by the solid line) shows an increase over time, demonstrating that viral evolution is ongoing despite the persistence of drug resistance mutations.
FIG. 7.
Schematic illustration of the first decade (approximately) of HIV infection following infection with a resistant strain of virus. Patients initially infected with a DR variant will typically demonstrate a transient high-titer viremia, followed by a spontaneous decline to a steady-state or “set point” viremia. During these first 6 months, bulk sequencing of plasma virus will typically detect only resistant virus in patients with transmitted DR virus. Years may pass during which the gradual process of random and potentially selective mutations results in the appearance of a mixture at the site(s) of a previous drug-resistant mutation(s) followed by ultimate “reversion” or, more accurately, replacement by WT virus. The resistant variant, however, will persist for the life of the patient, harbored within the reservoir of long-lived memory CD4 cells. ARV, antiretroviral.
Similar articles
- Persistence of primary drug resistance among recently HIV-1 infected adults.
Barbour JD, Hecht FM, Wrin T, Liegler TJ, Ramstead CA, Busch MP, Segal MR, Petropoulos CJ, Grant RM. Barbour JD, et al. AIDS. 2004 Aug 20;18(12):1683-9. doi: 10.1097/01.aids.0000131391.91468.ff. AIDS. 2004. PMID: 15280779 - Antiretroviral drug resistance mutations sustain or enhance CTL recognition of common HIV-1 Pol epitopes.
Mason RD, Bowmer MI, Howley CM, Gallant M, Myers JC, Grant MD. Mason RD, et al. J Immunol. 2004 Jun 1;172(11):7212-9. doi: 10.4049/jimmunol.172.11.7212. J Immunol. 2004. PMID: 15153547 - Persistence of wild-type virus and lack of temporal structure in the latent reservoir for human immunodeficiency virus type 1 in pediatric patients with extensive antiretroviral exposure.
Ruff CT, Ray SC, Kwon P, Zinn R, Pendleton A, Hutton N, Ashworth R, Gange S, Quinn TC, Siliciano RF, Persaud D. Ruff CT, et al. J Virol. 2002 Sep;76(18):9481-92. doi: 10.1128/jvi.76.18.9481-9492.2002. J Virol. 2002. PMID: 12186930 Free PMC article. - Study of the impact of HIV genotypic drug resistance testing on therapy efficacy.
Van Vaerenbergh K. Van Vaerenbergh K. Verh K Acad Geneeskd Belg. 2001;63(5):447-73. Verh K Acad Geneeskd Belg. 2001. PMID: 11813503 Review. - Drug-resistant human immunodefiency virus.
Kozal MJ. Kozal MJ. Clin Microbiol Infect. 2009 Jan;15 Suppl 1:69-73. doi: 10.1111/j.1469-0691.2008.02687.x. Clin Microbiol Infect. 2009. PMID: 19220361 Review.
Cited by
- Prevalence of Pretreatment HIV-1 Drug Resistance in Armenia in 2017-2018 and 2020-2021 following a WHO Survey.
Kirichenko A, Kireev D, Lapovok I, Shlykova A, Lopatukhin A, Pokrovskaya A, Ladnaya N, Grigoryan T, Petrosyan A, Sarhatyan T, Sargsyants N, Hovsepyan T, Ghazaryan H, Hovakimyan H, Martoyan S, Pokrovsky V. Kirichenko A, et al. Viruses. 2022 Oct 22;14(11):2320. doi: 10.3390/v14112320. Viruses. 2022. PMID: 36366418 Free PMC article. - Low prevalence of transmitted HIV type 1 drug resistance among antiretroviral-naive adults in a rural HIV clinic in Kenya.
Hassan AS, Mwaringa SM, Obonyo CA, Nabwera HM, Sanders EJ, Rinke de Wit TF, Cane PA, Berkley JA. Hassan AS, et al. AIDS Res Hum Retroviruses. 2013 Jan;29(1):129-35. doi: 10.1089/AID.2012.0167. Epub 2012 Sep 11. AIDS Res Hum Retroviruses. 2013. PMID: 22900472 Free PMC article. - National survey of pre-treatment HIV drug resistance in Cuban patients.
Machado LY, Blanco M, López LS, Díaz HM, Dubed M, Valdés N, Noa E, Martínez L, Pérez MT, Romay DM, Rivero CB, Joanes J, Cancio I, Lantero MI, Rodríguez M. Machado LY, et al. PLoS One. 2019 Sep 3;14(9):e0221879. doi: 10.1371/journal.pone.0221879. eCollection 2019. PLoS One. 2019. PMID: 31479466 Free PMC article. - HIV-1 reverse transcriptase drug-resistance mutations in chronically infected individuals receiving or naïve to HAART in Cameroon.
Burda ST, Viswanath R, Zhao J, Kinge T, Anyangwe C, Tinyami ET, Haldar B, Powell RL, Jarido V, Hewlett IK, Nyambi PN. Burda ST, et al. J Med Virol. 2010 Feb;82(2):187-96. doi: 10.1002/jmv.21677. J Med Virol. 2010. PMID: 20029816 Free PMC article. - Transmission of human immunodeficiency virus I drug resistance - a case report. What are the clinical implications?
Anadol E, Kaiser R, Verheyen J, Schülter E, Emmelkamp J, Schwarze-Zander C, Kupfer B, Wasmuth JC, Rockstroh JK. Anadol E, et al. Eur J Med Res. 2010 May 18;15(5):225-30. doi: 10.1186/2047-783x-15-5-225. Eur J Med Res. 2010. PMID: 20562063 Free PMC article.
References
- Barbour, J. D., F. M. Hecht, T. Wrin, T. J. Liegler, C. A. Ramstead, M. P. Busch, M. R. Segal, C. J. Petropoulos, and R. M. Grant. 2004. Persistence of primary drug resistance among recently HIV-1 infected adults. AIDS 181683-1689. - PubMed
- Brenner, B. G., J. P. Routy, M. Petrella, D. Moisi, M. Oliveira, M. Detorio, B. Spira, V. Essabag, B. Conway, R. Lalonde, R. P. Sekaly, and M. A. Wainberg. 2002. Persistence and fitness of multidrug-resistant human immunodeficiency virus type 1 acquired in primary infection. J. Virol. 761753-1761. - PMC - PubMed
- Colgrove, R. C., J. Pitt, P. H. Chung, S. L. Welles, and A. J. Japour. 1998. Selective vertical transmission of HIV-1 antiretroviral resistance mutations. AIDS 122281-2288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI68636/AI/NIAID NIH HHS/United States
- R01 AI057167/AI/NIAID NIH HHS/United States
- U01 AI043638-09/AI/NIAID NIH HHS/United States
- R01 AI047745/AI/NIAID NIH HHS/United States
- UM1 AI069432/AI/NIAID NIH HHS/United States
- U01 AI027670/AI/NIAID NIH HHS/United States
- R01 AI047745-06/AI/NIAID NIH HHS/United States
- R21 AI047745/AI/NIAID NIH HHS/United States
- AI29164/AI/NIAID NIH HHS/United States
- R56 AI047745/AI/NIAID NIH HHS/United States
- P30 AI036214/AI/NIAID NIH HHS/United States
- AI57167/AI/NIAID NIH HHS/United States
- P30 AI036214-13/AI/NIAID NIH HHS/United States
- AI43638/AI/NIAID NIH HHS/United States
- AI47745/AI/NIAID NIH HHS/United States
- AI38858/AI/NIAID NIH HHS/United States
- AI36214/AI/NIAID NIH HHS/United States
- R37 AI029164/AI/NIAID NIH HHS/United States
- U01 AI069432/AI/NIAID NIH HHS/United States
- AI27670/AI/NIAID NIH HHS/United States
- U01 AI038858/AI/NIAID NIH HHS/United States
- U01 AI043638/AI/NIAID NIH HHS/United States
- U01 AI068636/AI/NIAID NIH HHS/United States
- AI69432/AI/NIAID NIH HHS/United States
- R37 AI029164-15/AI/NIAID NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
- R24 AI106039/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases