Phosphorylation of sodium channel Na(v)1.8 by p38 mitogen-activated protein kinase increases current density in dorsal root ganglion neurons - PubMed (original) (raw)
Phosphorylation of sodium channel Na(v)1.8 by p38 mitogen-activated protein kinase increases current density in dorsal root ganglion neurons
Andy Hudmon et al. J Neurosci. 2008.
Abstract
The sensory neuron-specific sodium channel Na(v)1.8 and p38 mitogen-activated protein kinase are potential therapeutic targets within nociceptive dorsal root ganglion (DRG) neurons in inflammatory, and possibly neuropathic, pain. Na(v)1.8 channels within nociceptive DRG neurons contribute most of the inward current underlying the depolarizing phase of action potentials. Nerve injury and inflammation of peripheral tissues cause p38 activation in DRG neurons, a process that may contribute to nociceptive neuron hyperexcitability, which is associated with pain. However, how substrates of activated p38 contribute to DRG neuron hyperexcitability is currently not well understood. We report here, for the first time, that Na(v)1.8 and p38 are colocalized in DRG neurons, that Na(v)1.8 within DRG neurons is a substrate for p38, and that direct phosphorylation of the Na(v)1.8 channel by p38 regulates its function in these neurons. We show that direct phosphorylation of Na(v)1.8 at two p38 phospho-acceptor serine residues on the L1 loop (S551 and S556) causes an increase in Na(v)1.8 current density that is not accompanied by changes in gating properties of the channel. Our study suggests a mechanism by which activated p38 contributes to inflammatory, and possibly neuropathic, pain through a p38-mediated increase of Na(v)1.8 current density.
Figures
Figure 1.
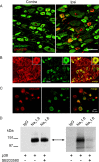
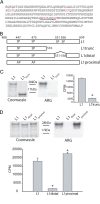
Native Nav1.8 sodium channels are colocalized and phosphorylated by p38 MAPK. A, CFA injection induces activated p38 in DRG neurons. Two days after CFA injection into the hindpaw, activated (phosphorylated) p38 immunolabeling (red) is exhibited predominantly in ipsilateral (right) compared with contralateral (left) DRG neurons. Peripherin (green) labels small-diameter neurons that project unmyelinated fibers. Colocalization of phosphorylated p38 and peripherin appears yellow. Scale bar, 50 μm. B, Nav1.8 (green) and phospho-p38 (red) immunolabeling in DRG neurons. The merged image demonstrates colocalization of Nav1.8 and phospho-p38 (yellow) in a subset of small- to medium-size DRG neurons. Scale bar, 50 μm. C, Phosphorylated p38 and Nav1.8 colocalization in anisomycin-treated DRG neurons. Phosphorylated p38 (red) and Nav1.8 (green) colocalization (yellow) is displayed in some DRG neurons, whereas other neurons exhibit only phosphorylated p38 or Nav1.8 immunolabeling. D, Native Nav1.8 is phosphorylated by activated p38. Lane 1 of the Western (left panel) and the ARG (right panel) shows that no protein product was immunoprecipitated nor phosphorylated by the control IgG antibody. Lanes 2 and 3 of the Western blot demonstrate that equal levels of immunoprecipitated Nav1.8 channel were used in the kinase assay. Lanes 2 and 3 of the ARG demonstrate that p38 phosphorylation of the Nav1.8 immunoprecipitated product (double-headed arrow) is only observed in the absence of the specific p38 inhibitor (SB203580; 10 μ
m
).
Figure 2.
Anisomycin increases peak current density of Nav1.8 in native rat DRG neurons. A, Representative families of current traces that were recorded in the presence of 0.3 μ
m
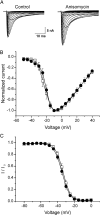
TTX are shown, in which cells were depolarized to a variety of potentials (−50 to +40 mV) from a holding potential of −70 mV to elicit Nav1.8 sodium current. Cells were treated with vehicle (DMSO; control) or anisomycin (10 μg/ml) for 30 min before recording. B, Mean normalized I–V curves for Nav1.8 currents pretreated with vehicle (□; _V_1/2 = −20.28 ± 0.26 mV; k = 5.28 ± 0.18; n = 12) or anisomycin (●; _V_1/2 = −19.42 ± 0.46 mV; k = 5.04 ± 0.32; n = 9) for 30 min revealed an identical voltage dependence of activation. C, Best-fit curves of steady-state fast inactivation were generated by Boltzmann distribution equation: vehicle (□; n = 9; _V_1/2 = −33.54 ± 0.65 mV; k = 4.86 ± 0.25); anisomycin (●; n = 8; _V_1/2 = −35.16 ± 0.73 mV; k = 5.10 ± 0.27). Error bars indicate SE.
Figure 3.
The specific p38 MAPK inhibitor SB203580 blocks anisomycin-mediated increase in Nav1.8 current density. A, Representative families of current traces that were recorded in the presence of 0.3 μ
m
TTX are shown, in which cells were depolarized to a variety of potentials (−50 to +40 mV) from a holding potential of −70 mV to elicit Nav1.8 sodium current. Cells were treated with anisomycin (10 μg/ml) for 30 min, the specific p38 MAP kinase inhibitor SB203580 (10 μ
m
) for 30 min followed by cotreatment with anisomycin for 30 min, or the inactive analog SB202474 (10 μ
m
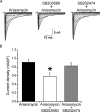
) for 30 min followed by cotreatment with anisomycin for 30 min. B, The increase in peak current density of Nav1.8 induced by anisomycin treatment is blocked by treatment with SB203580 but not by the inactive analog SB202474. *p < 0.05. Error bars indicate SE.
Figure 4.
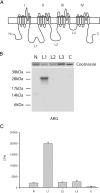
Nav1.8 L1 is phosphorylated by p38 MAPK. A, Schematic diagram illustrating the cytoplasmic fragments (N, 14 kDa; L1, 27 kDa; L2, 27 kDa; L3, 6 kDa; C terminus, 24 kDa) that were expressed in bacteria as 6×His-tagged fusion proteins and tested as substrates for p38. B, Autoradiogram illustrating that the L1 fusion protein was the only 32P-labeled substrate phosphorylated by p38 (see Materials and Methods). The relative levels of each fusion protein present in the kinase assay can be seen in the top panel as stained by Coomassie blue. C, Quantitative measurements of phosphorylation as assessed using the P81 filter assay and Cerenkov counting (n = 4; mean ± SD).
Figure 5.
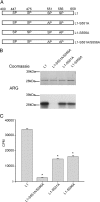
Identification of the p38 phosphorylation sites in L1 of Nav1.8. Deletion analysis and site-directed mutagenesis were used to identify the specific proline-directed serine residues required for p38 phosphorylation of L1. A, Amino acid sequence of the L1 region linking domains I and II shows four potential p38 phosphorylation sites (i.e., proline-directed serine or SP dipeptide; red lettering). B, Schematic diagram illustrating L1 (amino acids 400–659), L1-trunc (amino acids 400–516), L1-proximal (site-specific mutagenesis of S447 and S475 to alanine), and L1-distal (deletion of amino acids 551–558) that were expressed in bacteria as 6×His-tagged fusion proteins and used as substrates for p38 kinase assays. C, Autoradiogram illustrating that L1-trunc (deleting amino acids 517–659 in L1) is a poor substrate for p38 phosphorylation compared with L1 (right ARG). The relative level of each fusion protein used in the kinase assay is shown by Coomassie blue staining (left gel). Incorporation of 32P is determined as Cerenkov counts after p38 phosphorylation of L1 and L1-trunc (*p < 0.01; n = 4; mean ± SD). D, Autoradiogram illustrating that deleting amino acids 551–558 in L1 (L1-distal) disrupt p38 phosphorylation compared with L1 and mutation of the proximal proline-directed serine residues at 447 and 475 (right ARG). The relative level of each fusion protein in the kinase assay is shown by Coomassie blue staining (left gel). Incorporation of 32P is determined as Cerenkov counts after p38 phosphorylation of L1, L1-proximal, and L1-distal (*p < 0.01; n = 4; mean ± SD).
Figure 6.
S551 and S556 contribute equally to the p38 phosphorylation of L1 of Nav1.8. Site-directed mutagenesis of S551 and S556 (single and double mutants) was used to examine their relative contribution to p38 phosphorylation of L1. A, Schematic diagram illustrating L1 (amino acids 400–659), L1-S551A (serine 551 mutated to alanine), L1-S556A (serine 556 mutated to alanine), and L1-S551A/S556A (serine 551 and serine 556 mutated to alanines) that were expressed in bacteria as 6×His-tagged fusion proteins and used as substrates for p38. B, Autoradiogram illustrating that S551 and S556 both contribute to the p38 phosphorylation of L1 (bottom ARG). The relative levels of each fusion protein used in the kinase assay visualized by Coomassie blue (top gel). C, Cerenkov counts of p38 phosphorylation demonstrate that single- and double-mutant substrates are significantly less phosphorylated compared with WT (*p < 0.01; n = 4; mean ± SD).
Figure 7.
Phosphorylation of S551 and S556 in L1 of Nav1.8 by p38 mediates the increase in current density. A, Top panels, Untransfected Nav1.8−/− DRG neurons in culture for 24 h do not exhibit activated (phosphorylated) p38 immunolabeling, but an enhanced immunolabeling signal (red) from activated p38 is displayed by DRG neurons after the treatment with anisomycin. A, Bottom panels, transfected Nav1.8−/− DRG neurons in culture for 24 h exhibit activated p38 immunolabeling (red) both with and without anisomycin treatment. Scale bar, 50 μm. B, The current density of WT Nav1.8 in transfected Nav1.8−/− DRG neurons was significantly (*p < 0.05) smaller after treatment with SB203580 compared with SB202474. C, Peak current density of Nav1.8/S551–556A did not show significant reduction with SB203580 treatment compared with SB202474. Error bars indicate SE.
Similar articles
- ERK1/2 mitogen-activated protein kinase phosphorylates sodium channel Na(v)1.7 and alters its gating properties.
Stamboulian S, Choi JS, Ahn HS, Chang YW, Tyrrell L, Black JA, Waxman SG, Dib-Hajj SD. Stamboulian S, et al. J Neurosci. 2010 Feb 3;30(5):1637-47. doi: 10.1523/JNEUROSCI.4872-09.2010. J Neurosci. 2010. PMID: 20130174 Free PMC article. - Voltage-gated sodium channel Nav1.6 is modulated by p38 mitogen-activated protein kinase.
Wittmack EK, Rush AM, Hudmon A, Waxman SG, Dib-Hajj SD. Wittmack EK, et al. J Neurosci. 2005 Jul 13;25(28):6621-30. doi: 10.1523/JNEUROSCI.0541-05.2005. J Neurosci. 2005. PMID: 16014723 Free PMC article. - CC chemokine ligand 2 upregulates the current density and expression of TRPV1 channels and Nav1.8 sodium channels in dorsal root ganglion neurons.
Kao DJ, Li AH, Chen JC, Luo RS, Chen YL, Lu JC, Wang HL. Kao DJ, et al. J Neuroinflammation. 2012 Aug 8;9:189. doi: 10.1186/1742-2094-9-189. J Neuroinflammation. 2012. PMID: 22870919 Free PMC article. - Are voltage-gated sodium channels on the dorsal root ganglion involved in the development of neuropathic pain?
Wang W, Gu J, Li YQ, Tao YX. Wang W, et al. Mol Pain. 2011 Feb 23;7:16. doi: 10.1186/1744-8069-7-16. Mol Pain. 2011. PMID: 21345196 Free PMC article. Review. - The trafficking of Na(V)1.8.
Swanwick RS, Pristerá A, Okuse K. Swanwick RS, et al. Neurosci Lett. 2010 Dec 10;486(2):78-83. doi: 10.1016/j.neulet.2010.08.074. Epub 2010 Sep 15. Neurosci Lett. 2010. PMID: 20816723 Free PMC article. Review.
Cited by
- TNF-α acutely enhances acid-sensing ion channel currents in rat dorsal root ganglion neurons via a p38 MAPK pathway.
Wei S, Qiu CY, Jin Y, Liu TT, Hu WP. Wei S, et al. J Neuroinflammation. 2021 Apr 14;18(1):92. doi: 10.1186/s12974-021-02151-w. J Neuroinflammation. 2021. PMID: 33853615 Free PMC article. - Upregulation of miR-133a-3p in the Sciatic Nerve Contributes to Neuropathic Pain Development.
Chang LL, Wang HC, Tseng KY, Su MP, Wang JY, Chuang YT, Wang YH, Cheng KI. Chang LL, et al. Mol Neurobiol. 2020 Sep;57(9):3931-3942. doi: 10.1007/s12035-020-01999-y. Epub 2020 Jul 6. Mol Neurobiol. 2020. PMID: 32632603 - Unveiling Targets for Treating Postoperative Pain: The Role of the TNF-α/p38 MAPK/NF-κB/Nav1.8 and Nav1.9 Pathways in the Mouse Model of Incisional Pain.
de Lima FO, Lauria PSS, do Espírito-Santo RF, Evangelista AF, Nogueira TMO, Araldi D, Soares MBP, Villarreal CF. de Lima FO, et al. Int J Mol Sci. 2022 Oct 1;23(19):11630. doi: 10.3390/ijms231911630. Int J Mol Sci. 2022. PMID: 36232927 Free PMC article. - Monoacylglycerol Lipase Inhibitors Reverse Paclitaxel-Induced Nociceptive Behavior and Proinflammatory Markers in a Mouse Model of Chemotherapy-Induced Neuropathy.
Curry ZA, Wilkerson JL, Bagdas D, Kyte SL, Patel N, Donvito G, Mustafa MA, Poklis JL, Niphakis MJ, Hsu KL, Cravatt BF, Gewirtz DA, Damaj MI, Lichtman AH. Curry ZA, et al. J Pharmacol Exp Ther. 2018 Jul;366(1):169-183. doi: 10.1124/jpet.117.245704. Epub 2018 Mar 14. J Pharmacol Exp Ther. 2018. PMID: 29540562 Free PMC article. - Nicotine suppresses hyperexcitability of colonic sensory neurons and visceral hypersensivity in mouse model of colonic inflammation.
Abdrakhmanova GR, Kang M, Imad Damaj M, Akbarali HI. Abdrakhmanova GR, et al. Am J Physiol Gastrointest Liver Physiol. 2012 Apr;302(7):G740-7. doi: 10.1152/ajpgi.00411.2011. Epub 2012 Jan 12. Am J Physiol Gastrointest Liver Physiol. 2012. PMID: 22241859 Free PMC article.
References
- Akopian AN, Sivilotti L, Wood JN. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature. 1996;379:257–262. - PubMed
- Akopian AN, Souslova V, England S, Okuse K, Ogata N, Ure J, Smith A, Kerr BJ, McMahon SB, Boyce S, Hill R, Stanfa LC, Dickenson AH, Wood JN. The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways. Nat Neurosci. 1999;2:541–548. - PubMed
- Amaya F, Decosterd I, Samad TA, Plumpton C, Tate S, Mannion RJ, Costigan M, Woolf CJ. Diversity of expression of the sensory neuron-specific TTX-resistant voltage-gated sodium ion channels SNS and SNS2. Mol Cell Neurosci. 2000;15:331–342. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases