Clathrin dependence of synaptic-vesicle formation at the Drosophila neuromuscular junction - PubMed (original) (raw)
Clathrin dependence of synaptic-vesicle formation at the Drosophila neuromuscular junction
Heather Heerssen et al. Curr Biol. 2008.
Abstract
Background: Among the most prominent molecular constituents of a recycling synaptic vesicle is the clathrin triskelion, composed of clathrin light chain (Clc) and clathrin heavy chain (Chc). Remarkably, it remains unknown whether clathrin is strictly necessary for the stimulus-dependent re-formation of a synaptic vesicle and, conversely, whether clathrin-independent vesicle endocytosis exists at the neuronal synapse.
Results: We employ FlAsH-FALI-mediated protein photoinactivation to rapidly (3 min) and specifically disrupt Clc function at the Drosophila neuromuscular junction. We first demonstrate that Clc photoinactivation does not impair synaptic-vesicle fusion. We then provide electrophysiological and ultrastructural evidence that synaptic vesicles, once fused with the plasma membrane, cannot be re-formed after Clc photoinactivation. Finally, we demonstrate that stimulus-dependent membrane internalization occurs after Clc photoinactivation. However, newly internalized membrane fails to resolve into synaptic vesicles. Rather, newly internalized membrane forms large and extensive internal-membrane compartments that are never observed at a wild-type synapse.
Conclusions: We make three major conclusions. (1) FlAsH-FALI-mediated protein photoinactivation rapidly and specifically disrupts Clc function with no effect on synaptic-vesicle fusion. (2) Synaptic-vesicle re-formation does not occur after Clc photoinactivation. By extension, clathrin-independent "kiss-and-run" endocytosis does not sustain synaptic transmission during a stimulus train at this synapse. (3) Stimulus-dependent, clathrin-independent membrane internalization exists at this synapse, but it is unable to generate fusion-competent, small-diameter synaptic vesicles.
Figures
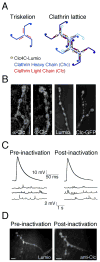
Figure 1. Clc4C expression and photoinactivation do not alter NMJ morphology or vesicle release
A) Diagram of a clathrin triskelion (left) and an assembled clathrin lattice upon which is diagrammed the approximate number and distribution of 4C epitope tags (yellow star). B) A wild-type NMJ (left) and an NMJ with neuronal expression of UAS-Clc4C (second from left; Clc4C) are stained with anti-Clc and imaged identically. Scale bar represents 5 μM. An NMJ with neuronal expression of UAS-Clc4C (third from left; Clc4C) is shown stained with Lumio. At far right is an NMJ expressing UAS-Clc-GFP. C) An NMJ expressing UAS-Clc4C in neurons is shown labeled with Lumio prior to (pre-inactivation) and following illumination (post-inactivation; stained with anti-Clc because the Lumio channel has been bleached to extinction). There is no apparent change in Clc distribution following photoinactivation. Scale bar represents 5 μM**. D)** Representative EPSPs (top) and spontaneous miniature release events (bottom) prior to (pre-inactivation) and following illumination (post-inactivation).
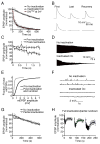
Figure 2. Clc4C photoinactivation prevents vesicle recycling
A) NMJs expressing UAS-Clc4C and labeled with Lumio received 6000 stimuli at 8 Hz following a 3 min period when the NMJ was either illuminated at 488 nm (Inactivated Clc) or not illuminated (No inactivation). 10 sequential EPSPs were averaged every 30 s, normalized to the initial average EPSP amplitude and plotted versus time. NMJs without photoinactivation sustained EPSP amplitudes while photoinactivated NMJs show pronounced synaptic depression until EPSPs are abolished. EPSP amplitudes recorded from shibirets2 mutant terminals, held at the non permissive temperature (34°C), show identical depression (red). B) Representative EPSPs from (A) including EPSPs sampled 5 s after cessation of the stimulus train. C) Quantification of EPSP amplitude during stimulation at 8 Hz in the same Lumio labeled Clc4C-expressing animals prior to (circles) and following (squares) illumination. D) Representative stimulus trains such as those used to generate the data in (A). E) Spontaneous miniature release event amplitude (mepsp) distributions are plotted for NMJs prior to photoinactivation and following photoinactivation and rundown (6000 stimuli at 8 Hz as in (A)). F) Representative mepsp traces from (E). G) Data were collected and plotted as in (A) except that the NMJs received 20 s of illumination at 488 nm (partial photoinactivation). H) Data are plotted as in (A). Following 3 min of illumination at 488 nm, NMJs received three successive stimulus trains: 100 stimuli at 20 Hz followed by recovery stimulation at 0.2 Hz; 200 stimuli at 20 Hz followed by recovery stimulation at 0.2 Hz; 500 stimuli at 20 Hz followed by recovery stimulation at 0.2 Hz. Data are presented as average (± SEM).
Figure 3. Stimulus-dependent membrane internalization without vesicle re-formation following Clc4C photoinactivation
A) A control NMJ (Lumio labeled, UAS-Clc4C expressing NMJ without photoinactivation) is shown. The NMJ was fixed following 6000 stimuli at 8 Hz and 1 min rest. NMJ ultrastructure is normal (n = 2 NMJ, sections from 10 boutons/NMJ). Insets 4× magnification. B) Panels B1-3 show representative sections of Lumio labeled UAS-Clc4C expressing NMJs following photoinactivation and stimulation as in (A). In panels B1 and B2 the synaptic bouton is filled with sheets of membrane that form large, elongated membrane compartments. In panel B3 a synaptic bouton is observed that is devoid of all synaptic vesicles. Control bouton profiles without synaptic vesicles were never observed. Scale bar = 500 nm.
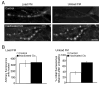
Figure 4. FM4-64 can be loaded but cannot be re-released following Clc4C photoinactivation
A) The top images show a control NMJ (UAS-Clc4C expressing, Lumio labeled NMJ, without photoinactivation) that received 6000 stimuli at 8 Hz in the presence of FM4-64. Efficient, stimulus-dependent loading of FM4-64 is observed (left) and greater than 60% of the FM4-64 can be unloaded from the nerve terminal following a second round of stimulation with high potassium saline lacking FM4-64 (right). Lower images show a UAS-Clc4C expressing NMJ that was Lumio labeled and photo-inactivated prior to stimulation in the presence of FM4-64. Efficient FM4-64 loading is observed (left). However, subsequent stimulation in the absence of FM4-64 fails to release significant amounts of internalized FM4-64. Scale bar represents 5 μM**. B)** Data from experiments as in (A) are quantified. There is no difference in the loading of FM4-64 comparing photoinactivated NMJs to control (left). There is significantly less FM4-64 unloading following FM4-64 photoinactivation (p < 0.01). Data represent average (± SEM).
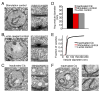
Figure 5. Altered membrane internalization following Clc4C photoinactivation and partial depletion of the synaptic vesicle pool
A) A representative example of a UAS-Clc4C expressing NMJ that was labeled with Lumio but not photoinactivated prior to stimulation (30 s at 50 Hz). NMJ ultrastructure is normal. B) A representative example of a UAS-Clc4C expressing NMJ that was Lumio labeled and photoinactivated without stimulation. NMJ ultrastructure is normal. C) A representative example of a UAS-Clc4C expressing NMJ that was Lumio labeled, illuminated for 3 min at 488 nm and stimulated (30 s, 50 Hz). The synaptic bouton contains numerous enlarged, circular membrane compartments. D) Quantification of the diameter of all vesicle-like structures (including synaptic vesicles) within a 500 nm radius of an active zone, defined as opposing pre- and postsynaptic densities with the presence of a T-bar structure and clustered synaptic vesicles. Experimental conditions are as described in (A). Photoinactivation results in a significant increase in average vesicle diameter (p < 0.001; error bars are too small to be reproduced on the graph). E) Distribution of vesicle diameters for data in (D). Control distributions superimpose. F) An NMJ from an experiment as in (C), where the NMJ received 3000 stimuli at 10 Hz, showing evidence of aberrant membrane internalization.
Similar articles
- Inactivation of clathrin heavy chain inhibits synaptic recycling but allows bulk membrane uptake.
Kasprowicz J, Kuenen S, Miskiewicz K, Habets RL, Smitz L, Verstreken P. Kasprowicz J, et al. J Cell Biol. 2008 Sep 8;182(5):1007-16. doi: 10.1083/jcb.200804162. Epub 2008 Sep 1. J Cell Biol. 2008. PMID: 18762582 Free PMC article. - FM 1-43 labeling of synaptic vesicle pools at the Drosophila neuromuscular junction.
Verstreken P, Ohyama T, Bellen HJ. Verstreken P, et al. Methods Mol Biol. 2008;440:349-69. doi: 10.1007/978-1-59745-178-9_26. Methods Mol Biol. 2008. PMID: 18369958 Free PMC article. - Drosophila endosomal proteins hook and deep orange regulate synapse size but not synaptic vesicle recycling.
Narayanan R, Krämer H, Ramaswami M. Narayanan R, et al. J Neurobiol. 2000 Nov 5;45(2):105-19. doi: 10.1002/1097-4695(20001105)45:2<105::aid-neu5>3.0.co;2-x. J Neurobiol. 2000. PMID: 11018772 - Synaptic vesicle endocytosis: the races, places, and molecular faces.
Morgan JR, Augustine GJ, Lafer EM. Morgan JR, et al. Neuromolecular Med. 2002;2(2):101-14. doi: 10.1385/NMM:2:2:101. Neuromolecular Med. 2002. PMID: 12428806 Review.
Cited by
- Slit diaphragm maintenance requires dynamic clathrin-mediated endocytosis facilitated by AP-2, Lap, Aux and Hsc70-4 in nephrocytes.
Wang L, Wen P, van de Leemput J, Zhao Z, Han Z. Wang L, et al. Cell Biosci. 2021 May 11;11(1):83. doi: 10.1186/s13578-021-00595-4. Cell Biosci. 2021. PMID: 33975644 Free PMC article. - Plasma membrane overgrowth causes fibrotic collagen accumulation and immune activation in Drosophila adipocytes.
Zang Y, Wan M, Liu M, Ke H, Ma S, Liu LP, Ni JQ, Pastor-Pareja JC. Zang Y, et al. Elife. 2015 Jun 19;4:e07187. doi: 10.7554/eLife.07187. Elife. 2015. PMID: 26090908 Free PMC article. - A postsynaptic PI3K-cII dependent signaling controller for presynaptic homeostatic plasticity.
Hauswirth AG, Ford KJ, Wang T, Fetter RD, Tong A, Davis GW. Hauswirth AG, et al. Elife. 2018 Jan 5;7:e31535. doi: 10.7554/eLife.31535. Elife. 2018. PMID: 29303480 Free PMC article. - Acute increase of α-synuclein inhibits synaptic vesicle recycling evoked during intense stimulation.
Busch DJ, Oliphint PA, Walsh RB, Banks SM, Woods WS, George JM, Morgan JR. Busch DJ, et al. Mol Biol Cell. 2014 Dec 1;25(24):3926-41. doi: 10.1091/mbc.E14-02-0708. Epub 2014 Oct 1. Mol Biol Cell. 2014. PMID: 25273557 Free PMC article. - The BLOC-1 Subunit Pallidin Facilitates Activity-Dependent Synaptic Vesicle Recycling.
Chen X, Ma W, Zhang S, Paluch J, Guo W, Dickman DK. Chen X, et al. eNeuro. 2017 Feb 8;4(1):ENEURO.0335-16.2017. doi: 10.1523/ENEURO.0335-16.2017. eCollection 2017 Jan-Feb. eNeuro. 2017. PMID: 28317021 Free PMC article.
References
- Brodsky FM, Chen CY, Knuehl C, Towler MC, Wakeham DE. Biological basket weaving: formation and function of clathrin-coated vesicles. Annu Rev Cell Dev Biol. 2001;17:517–568. - PubMed
- Ford MG, Mills IG, Peter BJ, Vallis Y, Praefcke GJ, Evans PR, McMahon HT. Curvature of clathrin-coated pits driven by epsin. Nature. 2002;419:361–366. - PubMed
- Heuser J. The role of coated vesicles in recycling of synaptic vesicle membrane. Cell Biol Int Rep. 1989;13:1063–1076. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous