Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior - PubMed (original) (raw)
Review
Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior
Dennis J Selkoe. Behav Brain Res. 2008.
Abstract
During the last 25 years, neuropathological, biochemical, genetic, cell biological and even therapeutic studies in humans have all supported the hypothesis that the gradual cerebral accumulation of soluble and insoluble assemblies of the amyloid beta-protein (Abeta) in limbic and association cortices triggers a cascade of biochemical and cellular alterations that produce the clinical phenotype of Alzheimer's disease (AD). The reasons for elevated cortical Abeta42 levels in most patients with typical, late-onset AD are unknown, but based on recent work, these could turn out to include augmented neuronal release of Abeta during some kinds of synaptic activity. Elevated levels of soluble Abeta42 monomers enable formation of soluble oligomers that can diffuse into synaptic clefts. We have identified certain APP-expressing cultured cell lines that form low-n oligomers intracellularly and release a portion of them into the medium. We find that these naturally secreted soluble oligomers--at picomolar concentrations--can disrupt hippocampal LTP in slices and in vivo and can also impair the memory of a complex learned behavior in rats. Abeta trimers appear to be more potent in disrupting LTP than are dimers. The cell-derived oligomers also decrease dendritic spine density in organotypic hippocampal slice cultures, and this decrease can be prevented by administration of Abeta antibodies or small-molecule modulators of Abeta aggregation. This therapeutic progress has been accompanied by advances in imaging the Abeta deposits non-invasively in humans. A new diagnostic-therapeutic paradigm to successfully address AD and its harbinger, mild cognitive impairment-amnestic type, is emerging.
Figures
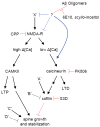
Figure 1
Proposed pathways that regulate spine density and that are affected by Aβ oligomers, based on the results of our results. Ca2+ influx through synaptic NMDARs can activate at least two pathways that regulate spine density. On the left-hand side, high levels of Ca2+ accumulation, such as those reached during tetanic or suprathreshold synaptic stimulation, induce LTP via a CAMKII-dependent pathway (reviewed in [44]). LTP-inducing stimuli also trigger the enlargement of dendritic spines and growth of new spines in a NMDAR- and CAMKII-dependent manner [13, 26, 35, 37, 42]. Introduction of active CAMKII in neurons is sufficient to induce new spine growth [26]. In the right-hand side pathway, low levels of Ca2+ accumulation, such as those reached during low-frequency subthreshold stimulation, induce LTD through a calcineurin-dependent pathway (reviewed in [34]. LTD-inducing stimuli also lead to spine shrinkage via an NMDAR/calcineurin/cofilin-dependent pathway and spine retraction through an NMDAR-dependent pathway [42, 72]. The calcineurin and cofilin dependence of LTD-associated spine retraction have not been examined. In this model, full block of NMDARs interrupts both pathways, leading to no net spine loss. Partial block of NMDARs favors activation of the right-hand pathway, LTD induction, and loss of spines. In addition, multiple factors (A, B, C, and D) act independently of NMDARs, CAMKII, and calcineurin to regulate cofilin and spine density. We find that soluble Aβ oligomers decrease spine density in an NMDAR/calcineurin/cofilin-dependent manner, consistent with activation of the pathway shown on the right. Aβ oligomers reduce NMDAR-dependent Ca2+ transients, possibly shifting stimuli that normally activate the left-hand pathway to instead activate those on the right. This activation might occur through direct interaction of Aβ with NMDARs or by first activating unknown factors (X) that may lead to inhibition of NMDAR-mediated synaptic Ca2+ influx. Aβ may also facilitate NMDAR-dependent activation of calcineurin via additional pathways. Blue lines indicate levels at which soluble Aβ oligomers may modulate the pathway and red lines indicate elements of the pathway tested in this study.
Similar articles
- Human Brain-Derived Aβ Oligomers Bind to Synapses and Disrupt Synaptic Activity in a Manner That Requires APP.
Wang Z, Jackson RJ, Hong W, Taylor WM, Corbett GT, Moreno A, Liu W, Li S, Frosch MP, Slutsky I, Young-Pearse TL, Spires-Jones TL, Walsh DM. Wang Z, et al. J Neurosci. 2017 Dec 6;37(49):11947-11966. doi: 10.1523/JNEUROSCI.2009-17.2017. Epub 2017 Nov 3. J Neurosci. 2017. PMID: 29101243 Free PMC article. - Effects of secreted oligomers of amyloid beta-protein on hippocampal synaptic plasticity: a potent role for trimers.
Townsend M, Shankar GM, Mehta T, Walsh DM, Selkoe DJ. Townsend M, et al. J Physiol. 2006 Apr 15;572(Pt 2):477-92. doi: 10.1113/jphysiol.2005.103754. Epub 2006 Feb 9. J Physiol. 2006. PMID: 16469784 Free PMC article. - Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory.
Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Shankar GM, et al. Nat Med. 2008 Aug;14(8):837-42. doi: 10.1038/nm1782. Epub 2008 Jun 22. Nat Med. 2008. PMID: 18568035 Free PMC article. - Synaptic changes in Alzheimer's disease and its models.
Pozueta J, Lefort R, Shelanski ML. Pozueta J, et al. Neuroscience. 2013 Oct 22;251:51-65. doi: 10.1016/j.neuroscience.2012.05.050. Epub 2012 Jun 9. Neuroscience. 2013. PMID: 22687952 Review. - Dendritic spine loss and synaptic alterations in Alzheimer's disease.
Knobloch M, Mansuy IM. Knobloch M, et al. Mol Neurobiol. 2008 Feb;37(1):73-82. doi: 10.1007/s12035-008-8018-z. Epub 2008 Apr 26. Mol Neurobiol. 2008. PMID: 18438727 Review.
Cited by
- Natural Products and Their Neuroprotective Effects in Degenerative Brain Diseases: A Comprehensive Review.
Lim DW, Lee JE, Lee C, Kim YT. Lim DW, et al. Int J Mol Sci. 2024 Oct 18;25(20):11223. doi: 10.3390/ijms252011223. Int J Mol Sci. 2024. PMID: 39457003 Free PMC article. Review. - JNK inhibitor and ferroptosis modulator as possible therapeutic modalities in Alzheimer disease (AD).
Zakaria S, Ibrahim N, Abdo W, E El-Sisi A. Zakaria S, et al. Sci Rep. 2024 Oct 7;14(1):23293. doi: 10.1038/s41598-024-73596-1. Sci Rep. 2024. PMID: 39375359 Free PMC article. - The duality of amyloid-β: its role in normal and Alzheimer's disease states.
Azargoonjahromi A. Azargoonjahromi A. Mol Brain. 2024 Jul 17;17(1):44. doi: 10.1186/s13041-024-01118-1. Mol Brain. 2024. PMID: 39020435 Free PMC article. Review. - Somatostatin: Linking Cognition and Alzheimer Disease to Therapeutic Targeting.
Sandoval KE, Witt KA. Sandoval KE, et al. Pharmacol Rev. 2024 Oct 16;76(6):1291-1325. doi: 10.1124/pharmrev.124.001117. Pharmacol Rev. 2024. PMID: 39013601 Review. - Changes in hippocampal volume, synaptic plasticity and amylin sensitivity in an animal model of type 2 diabetes are associated with increased vulnerability to amyloid-beta in advancing age.
Tarhan M, Hartl T, Shchyglo O, Colitti-Klausnitzer J, Kuhla A, Breuer TM, Manahan-Vaughan D. Tarhan M, et al. Front Aging Neurosci. 2024 Jun 21;16:1373477. doi: 10.3389/fnagi.2024.1373477. eCollection 2024. Front Aging Neurosci. 2024. PMID: 38974903 Free PMC article.
References
- Bitan G, Teplow DB. Preparation of aggregate-free, low molecular weight amyloid-beta for assembly and toxicity assays. Methods Mol Biol. 2005;299:3–9. - PubMed
- Bloodgood BL, Sabatini BL. Nonlinear regulation of unitary synaptic signals by CaV(2.3) voltage-sensitive calcium channels located in dendritic spines. Neuron. 2007;53:249–260. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical