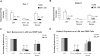Notch-dependent control of myelopoiesis is regulated by fucosylation - PubMed (original) (raw)
Notch-dependent control of myelopoiesis is regulated by fucosylation
Lan Zhou et al. Blood. 2008.
Abstract
Cell-cell contact-dependent mechanisms that modulate proliferation and/or differentiation in the context of hematopoiesis include mechanisms characteristic of the interactions between members of the Notch family of signal transduction molecules and their ligands. Whereas Notch family members and their ligands clearly modulate T lymphopoietic decisions, evidence for their participation in modulating myelopoiesis is much less clear, and roles for posttranslational control of Notch-dependent signal transduction in myelopoiesis are unexplored. We report here that a myeloproliferative phenotype in FX(-/-) mice, which are conditionally deficient in cellular fucosylation, is consequent to loss of Notch-dependent signal transduction on myeloid progenitor cells. In the context of a wild-type fucosylation phenotype, we find that the Notch ligands suppress myeloid differentiation of progenitor cells and enhance expression of Notch target genes. By contrast, fucosylation-deficient myeloid progenitors are insensitive to the suppressive effects of Notch ligands on myelopoiesis, do not transcribe Notch1 target genes when cocultured with Notch ligands, and have lost the wild-type Notch ligand-binding phenotype. Considered together, these observations indicate that Notch-dependent signaling controls myelopoiesis in vivo and in vitro and identifies a requirement for Notch fucosylation in the expression of Notch ligand binding activity and Notch signaling efficiency in myeloid progenitors.
Figures
Figure 1
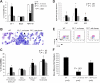
FX−/− mice develop a myeloproliferative disorder. (A) FACS analysis of the percentage of granulocytes (Gr-1+), B lymphocytes (B220+), and erythrocytes (TER119+) in the bone marrow in 3- to 4-month-old control mice (WT; n = 8), FX−/− mice reared on fucose-supplemented chow until 12 weeks of age, and then on standard chow for at least 4 weeks (n = 8; FX−/−, no fucose), and FX−/− mice reared on fucose-supplemented chow until use (n = 8; FX−/−, with fucose). (B) May-Grünwald-Giemsa–stained cytospins of marrow cells showing increased segmented neutrophils ( ) and myeloid progenitor cells (
) and myeloid progenitor cells ( ) in the bone marrow of FX−/− mice (no fucose). (C) Differential blood counts on May-Grünwald-Giemsa–stained cytospins of marrow cells showing percentage of myeloid progenitor cells at various developmental stage and mature neutrophils (WT, n = 8; FX−/−, no fucose, n = 9; FX−/−, with fucose, n = 9). (D,E) FACS analysis of bone marrow myeloid progenitors (CMP: Lin−c-kit+Sca-1−IL7R−CD34+FcγRIIlow; GMP: Lin−c-kit+Sca-1−IL7R−CD34+FcγRII+; MEP: Lin−c-kit+Sca-1−IL7R−CD34lowFcγRIIlow). (F) FACS analysis of CLP compartment (Lin−c-kitlowSca-1lowIL7R+). Bar graphs represent the average total number of CLP cells (± SD) from 2 tibias and 2 femurs of each mouse.
) in the bone marrow of FX−/− mice (no fucose). (C) Differential blood counts on May-Grünwald-Giemsa–stained cytospins of marrow cells showing percentage of myeloid progenitor cells at various developmental stage and mature neutrophils (WT, n = 8; FX−/−, no fucose, n = 9; FX−/−, with fucose, n = 9). (D,E) FACS analysis of bone marrow myeloid progenitors (CMP: Lin−c-kit+Sca-1−IL7R−CD34+FcγRIIlow; GMP: Lin−c-kit+Sca-1−IL7R−CD34+FcγRII+; MEP: Lin−c-kit+Sca-1−IL7R−CD34lowFcγRIIlow). (F) FACS analysis of CLP compartment (Lin−c-kitlowSca-1lowIL7R+). Bar graphs represent the average total number of CLP cells (± SD) from 2 tibias and 2 femurs of each mouse.
Figure 2
In vivo reconstitution of myeloid and lymphoid lineages using marrow cells from WT or FX−/− mice injected into FX−/− and WT recipients, and marrow cells from Fuc-T−/− mice injected into Fuc-T−/− recipients. (A) Schematic representation of the transplantation protocol. (B,C) Analysis of peripheral neutrophils (Gr-1+) and lymphocytes (B220+ and CD3ϵ+), and bone marrow myeloid progenitor cells by FACS 4 months after transplantation. Data are means plus or minus SD. Student t test was performed to compare the neutrophil or lymphocyte numbers in each transplant setting with those of WT to WT. P > .5 unless otherwise indicated. (D) One month after transplantation, WT recipient mice receiving donor cells from WT or FX−/− mice were injected intraperitoneally with BrdU and fed with water containing BrdU for 3 days. The percentage of CMP and GMP cells (Lin−c-kit+Sca-1−IL7R−CD34+; numbers on graphs) in S-G2/M phase of the cell cycle was analyzed by anti-BrdU and 7-amino-actinomycin D.
Figure 3
Stromal cells expressing Notch ligand block myelopoiesis in vitro. (A) Schematic representation of the in vitro coculture of CMP cells with OP9 cells expressing Notch ligands with cytokine cocktails. (B) Day 14 culture of bone marrow–derived CMP cells, cocultured with OP9-control cells (no ligand) or OP9 cells expressing Notch ligands, were analyzed for Gr-1 expression. Data are mean values of at least 4 determinations. The bar graph represents the percentage of Gr-1+ and Gr-1− cells among total viable cells. (C) May-Grünwald-Giemsa–stained cytospins of day 14 cultured cells with OP9-control, OP9-Jagged1, and OP9-Dll1. (D) Short-term culture (60 hours) of CMP cells with SCF was analyzed for the percentages of GMP and MEP generated from CMP (numbers shown to right of graphs). Data are representative of at least 3 independent experiments. (E) Myelopoiesis transcription factors (PU.1 and C/EBPα) were differentially expressed in OP9-Dll1 versus OP9-control cultures. Results (means ± SD; n = 4) have been standardized for GAPDH levels and were expressed as percentage of expression relative to the levels detected in OP9-control (set at 1). aTotal number of cells present at the end of culture with 500 cells input grown on OP9 stromal cells with SCF (50 ng/mL), IL-3 (5 ng/mL), Flt3L (10 ng/mL), and GM-CSF (2.5 ng/mL). Data are means plus or minus SD of 6 independent experiments (*P < .05, #P < .01).
Figure 4
GSI reverses the Notch target gene expression and blocked myeloid differentiation induced by Notch ligand in OP9 culture. (A) Expression of Hes1 and Deltex1 was analyzed by quantitative RT-PCR from 4-day CMP culture on OP9-Dll1 cells relative to the levels detected in OP9-control (set at 1) in the absence (Dll1) or the presence of 10 μM GSI (Dll1, GSI). (B) Day 14 culture of bone marrow–derived CMP cells, cocultured with OP9-control cells (no ligand) or OP9 cells expressing Notch ligand, in the absence or presence of 10 μM GSI, was analyzed for Gr-1 expression. Data are mean values (± SD) of at least 4 determinations and expressed as percentage of Gr-1+ cells relative to that from WT cells grown on OP9-control (no GSI; set at 100%).
Figure 5
Notch pathway–induced myeloid development block is fucosylation dependent. Expression of Gr-1 from day 14 culture CMP cells derived from FX−/− mice maintained on standard chow (FX−/−, no fucose; B) or maintained on fucose-supplemented chow at least 4 weeks and with 1 mM fucose in the culture medium (C), was compared with WT CMP culture on OP9-control and OP9-Dll1 (A), in the absence or presence of 10 μM GSI. Data are mean values (± SD) of at least 4 determinations and expressed as percentages of Gr-1+ cells relative to that on OP9-control (no GSI; set at 100%). Cells from panels B and C cultured on OP9-control and OP9-Dll1 were examined by May-Grünwald-Giemsa staining and FACS analysis of CMP to GMP conversion (D,E). Numbers on plots are percentages of the indicated cell types. (F) Day 14 culture of marrow CMP cells derived from WT, FX−/− mice maintained on standard chow (FX−/−, no fucose), and mice with double deficiency for the 2 alpha1,3fucosyltransferases Fuc-TIV−/−Fuc-TVII−/− (Fuc-T−/−), cocultured with OP9-control cells (no ligand) or OP9 cells expressing Notch ligands, was analyzed for Gr-1 expression. Data are mean values (± SD) of at least 4 determinations and expressed as percentage of Gr-1+ cells relative to that from cells grown on OP9-control (set at 100%). Student t test was performed to compare the parameters obtained with GSI to those without GSI (A-C) or parameters obtained from Fuc-T−/− and FX−/− mice to those from WT mice (F).
Figure 6
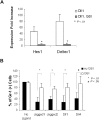
Expression of Notch target genes in FX−/− mice marrow cells in vitro and in vivo. Expression of Hes1 (A) and Deltex1 (B) from 4-day CMP (WT; FX−/−, no fucose; FX−/−, with fucose) cultured on OP9-Dll1 was compared with OP9-control cells (set at 1) in the absence or the presence of 10 μM GSI, expressed as fold increase. (C,D) Hes1 and Deltex1 expression was analyzed from freshly isolated LSK and CMP cells from WT, FX−/− mice maintained on standard chow (FX−/−, no fucose) and FX−/− mice maintained on fucose-supplement chow (FX−/−, with fucose). Data are means plus or minus SD of 4 determinations. Student t test was performed to compare the parameters obtained with GSI to those without GSI (A,B) or values obtained from FX−/− mice to those from WT mice (C,D).
Figure 7
Fucosylation-dependent binding of Notch ligands to FX−/− mice myeloid progenitors. Flow cytometric analysis of binding of recombinant Notch ligands Dll1 (1.3 μg/mL), Dll4 (0.5 μg/mL), Dll3 (0.3 μg/mL), and control (human IgG1 Fc, 1 μg/mL) to CMP cells isolated from WT mice, from FX−/− mice reared in the presence of fucose, or from FX−/− mice reared in the absence of fucose. Chelation of Ca2+ with ethylenediaminetetraacetic acid abolished binding (data not shown). Data are the flow cytometric histogram of experiment 1 (top panel) and mean fluorescence intensity (MFI) of the binding of CMPs with control and Notch ligands in 3 independent experiments (bottom panel).
Comment in
- Unsweetened Notch leads to myeloproliferation.
Haltiwanger RS. Haltiwanger RS. Blood. 2008 Jul 15;112(2):214-5. doi: 10.1182/blood-2008-04-150391. Blood. 2008. PMID: 18606881 Free PMC article.
Similar articles
- O-fucose modulates Notch-controlled blood lineage commitment.
Yan Q, Yao D, Wei LL, Huang Y, Myers J, Zhang L, Xin W, Shim J, Man Y, Petryniak B, Gerson S, Lowe JB, Zhou L. Yan Q, et al. Am J Pathol. 2010 Jun;176(6):2921-34. doi: 10.2353/ajpath.2010.090702. Epub 2010 Apr 2. Am J Pathol. 2010. PMID: 20363915 Free PMC article. - Protein O-fucosyltransferase 1 (Pofut1) regulates lymphoid and myeloid homeostasis through modulation of Notch receptor ligand interactions.
Yao D, Huang Y, Huang X, Wang W, Yan Q, Wei L, Xin W, Gerson S, Stanley P, Lowe JB, Zhou L. Yao D, et al. Blood. 2011 May 26;117(21):5652-62. doi: 10.1182/blood-2010-12-326074. Epub 2011 Apr 4. Blood. 2011. PMID: 21464368 Free PMC article. - Fucosylation Deficiency in Mice Leads to Colitis and Adenocarcinoma.
Wang Y, Huang D, Chen KY, Cui M, Wang W, Huang X, Awadellah A, Li Q, Friedman A, Xin WW, Di Martino L, Cominelli F, Miron A, Chan R, Fox JG, Xu Y, Shen X, Kalady MF, Markowitz S, Maillard I, Lowe JB, Xin W, Zhou L. Wang Y, et al. Gastroenterology. 2017 Jan;152(1):193-205.e10. doi: 10.1053/j.gastro.2016.09.004. Epub 2016 Sep 14. Gastroenterology. 2017. PMID: 27639802 Free PMC article. - Regulation of Notch signaling during T- and B-cell development by O-fucose glycans.
Stanley P, Guidos CJ. Stanley P, et al. Immunol Rev. 2009 Jul;230(1):201-15. doi: 10.1111/j.1600-065X.2009.00791.x. Immunol Rev. 2009. PMID: 19594638 Review. - Myeloproliferation and hematopoietic stem cell dysfunction due to defective Notch receptor modification by O-fucose glycans.
Zhou L. Zhou L. Semin Immunopathol. 2012 May;34(3):455-69. doi: 10.1007/s00281-012-0303-2. Epub 2012 Mar 14. Semin Immunopathol. 2012. PMID: 22415200 Review.
Cited by
- Notch Receptor-Ligand Engagement Maintains Hematopoietic Stem Cell Quiescence and Niche Retention.
Wang W, Yu S, Zimmerman G, Wang Y, Myers J, Yu VW, Huang D, Huang X, Shim J, Huang Y, Xin W, Qiao P, Yan M, Xin W, Scadden DT, Stanley P, Lowe JB, Huang AY, Siebel CW, Zhou L. Wang W, et al. Stem Cells. 2015 Jul;33(7):2280-93. doi: 10.1002/stem.2031. Epub 2015 May 13. Stem Cells. 2015. PMID: 25851125 Free PMC article. - Negative regulation of notch signaling by xylose.
Lee TV, Sethi MK, Leonardi J, Rana NA, Buettner FF, Haltiwanger RS, Bakker H, Jafar-Nejad H. Lee TV, et al. PLoS Genet. 2013 Jun;9(6):e1003547. doi: 10.1371/journal.pgen.1003547. Epub 2013 Jun 6. PLoS Genet. 2013. PMID: 23754965 Free PMC article. - Notch activation inhibits AML growth and survival: a potential therapeutic approach.
Kannan S, Sutphin RM, Hall MG, Golfman LS, Fang W, Nolo RM, Akers LJ, Hammitt RA, McMurray JS, Kornblau SM, Melnick AM, Figueroa ME, Zweidler-McKay PA. Kannan S, et al. J Exp Med. 2013 Feb 11;210(2):321-37. doi: 10.1084/jem.20121527. Epub 2013 Jan 28. J Exp Med. 2013. PMID: 23359069 Free PMC article. - Human deficiencies of fucosylation and sialylation affecting selectin ligands.
Lühn K, Wild MK. Lühn K, et al. Semin Immunopathol. 2012 May;34(3):383-99. doi: 10.1007/s00281-012-0304-1. Epub 2012 Mar 31. Semin Immunopathol. 2012. PMID: 22461019 Review. - Slc35c2 promotes Notch1 fucosylation and is required for optimal Notch signaling in mammalian cells.
Lu L, Hou X, Shi S, Körner C, Stanley P. Lu L, et al. J Biol Chem. 2010 Nov 12;285(46):36245-54. doi: 10.1074/jbc.M110.126003. Epub 2010 Sep 13. J Biol Chem. 2010. PMID: 20837470 Free PMC article.
References
- Elliott MA. Chronic neutrophilic leukemia: a contemporary review. Curr Hematol Rep. 2004;3:210–217. - PubMed
- Opdenakker G, Fibbe WE, Van Damme J. The molecular basis of leukocytosis. Immunol Today. 1998;19:182–189. - PubMed
- Jagels MA, Hugli TE. Mechanisms and mediators of neutrophilic leukocytosis. Immunopharmacology. 1994;28:1–18. - PubMed
- Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–672. - PubMed
- Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases