Building the actin cytoskeleton: filopodia contribute to the construction of contractile bundles in the lamella - PubMed (original) (raw)
Building the actin cytoskeleton: filopodia contribute to the construction of contractile bundles in the lamella
Maria Nemethova et al. J Cell Biol. 2008.
Abstract
Filopodia are rodlike extensions generally attributed with a guidance role in cell migration. We now show in fish fibroblasts that filopodia play a major role in generating contractile bundles in the lamella region behind the migrating front. Filopodia that developed adhesion to the substrate via paxillin containing focal complexes contributed their proximal part to stress fiber assembly, and filopodia that folded laterally contributed to the construction of contractile bundles parallel to the cell edge. Correlated light and electron microscopy of cells labeled for actin and fascin confirmed integration of filopodia bundles into the lamella network. Inhibition of myosin II did not subdue the waving and folding motions of filopodia or their entry into the lamella, but filopodia were not then integrated into contractile arrays. Comparable results were obtained with B16 melanoma cells. These and other findings support the idea that filaments generated in filopodia and lamellipodia for protrusion are recycled for seeding actomyosin arrays for use in retraction.
Figures
Figure 1.
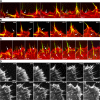
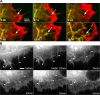
Examples of actin cytoskeleton phenotypes in fish fibroblasts showing differences in the lamella region (LM) behind the anterior zone containing lamellipodia (LP) and filopodia (FP). Cells were transfected with mCherry-actin. Images show single frames from video sequences. (A) Lamella with bundles parallel to the cell front, together with arc shaped segments (AC). (B) Lamella with stress fiber bundles (SF) mainly perpendicular to the cell front. See Videos 1 and 2 (available at
http://www.jcb.org/cgi/content/full/jcb.200709134/DC1
). Bar, 10 μm.
Figure 2.
Variable fates of filopodia. (A and B) Development of actin bundles in the lamella from lateral folding of filopodia into the base of the lamellipodium (transitions marked with arrows). Cells were transfected with mCherry-actin (red) and EGFP-fascin (green; see Video 3, available at
http://www.jcb.org/cgi/content/full/jcb.200709134/DC1
). Times are given in minutes and seconds. (C) Folding into, kinking (top arrow), and withdrawal (bottom arrow) of filopodia into lamella. The same cell as in B is shown. See Video 3. (D) Transition of radially oriented filopodia into stress fiber bundles. The filopodia marked 1–4 at time 5:40 remain essentially stationary as the cell front advances and finally appear as bundles in the lamella (30:00). Arrowheads mark equivalent positions on the filopodia through the sequence. At the position marked, the filopodia become kinked and separate from the lamellipodium/filopodium boundary, except filopodium 2, which continues extending and contributes a further bundle to the lamella. Filopodium 5 (5:40) fuses with two other filopodia; the resulting filopodium subsequently bends in two places and flows into the lamella (26:20; see Video 1). Bars, 5 μm.
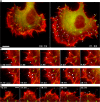
Figure 3.
Entry of filopodia into ventral layer of the cytoskeleton. (A and B) Simultaneous wide-field (red) and TIRF (green) microscopy of a cell expressing EGFP-fascin. Filopodia fold down into the zone of the evanescent wave to within 200 nm from the substrate. (A) Filopodium marked with arrowhead folds upwards and then backward into the cell; filopodia marked with arrows fold laterally and down into the cell edge. (B) The numbered filopodia fold in opposite directions into the cell edge. See Video 4 (available at
http://www.jcb.org/cgi/content/full/jcb.200709134/DC1
). (C) Periphery of a cell transfected with EGFP-fascin (pseudocolor red) and mCherry-actin (pseudocolor green) imaged simultaneously by TIRF microscopy. Note the generation of a ventral stress fiber bundle (arrow) from filopodia that fold bilaterally into the cell edge. Times are given in minutes and seconds. Bars, 5 μm.
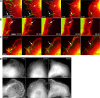
Figure 4.
Correlative live cell imaging and electron microscopy of filopodia transitions. (A) Image of a cell transfected with EGFP-fascin (green) and mCherry-actin (red) after fixation at the end of the video sequence. (B) Electron micrograph of region shown in A after negative staining. Boxes and arrows in A and B indicate regions corresponding to the video sequences shown in C and D. Arrows in C and D indicate transition steps of filopodia into lamella, with the eventual depletion of fascin; final panels show actin label alone in the fixed cell. Times are given in minutes and seconds. See Video 5 (available at
http://www.jcb.org/cgi/content/full/jcb.200709134/DC1
).
Figure 5.
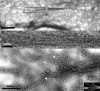
Integration of filopodia into lamella cytoskeleton. (A) Electron micrograph of region corresponding to the terminal frame in Fig. 4 C. (B) Enlargement of A (small box) showing central region of filopodium bundle in the lamella. (C) End region of the filopodia bundle (large box in A) showing splaying of filaments into adjacent bundles of the lamella (indicated by white arrows; also depicted in the inset). See Fig. S2 (available at
http://www.jcb.org/cgi/content/full/jcb.200709134/DC1
).
Figure 6.
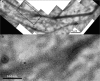
Integration of filopodia into lamella cytoskeleton. (A) Electron micrograph of the two bundles (1 and 2) marked by arrows in the terminal frame of Fig. 4 D. (B) Enlargement of the region boxed in A showing interconnection of the filopodium bundle with adjacent bundles of the lamella cytoskeleton. See also Fig. S3 (available at
http://www.jcb.org/cgi/content/full/jcb.200709134/DC1
).
Figure 7.
Coupling with myosin in the lamella. (A) Withdrawal and integration of a filopodium into actomyosin network of lamella. Cell was transfected with mCherry-actin and EGFP-myosin regulatory light chain. Arrows indicate a filopodium that first became kinked and was then drawn into the lamella with the progressive accumulation of myosin and the eventual transition into a stress fiber bundle. Times are given in minutes and seconds. See Video 6 (available at
http://www.jcb.org/cgi/content/full/jcb.200709134/DC1
). (B) Inhibition of myosin contractility with 50 μM blebbistatin. CAR fibroblast transfected with mCherry-actin. Filopodia formation was not inhibited and filopodia translocated into the lamella (arrowheads) but did not form contractile bundles. See Video 7.
Figure 8.
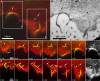
A subpopulation of filopodia are involved in the initiation of substrate adhesion. (A) Two video frames of a CAR fibroblast expressing mCherry-actin and EGFP-paxillin separated by 21 min and showing (marked on the right with arrowheads) those adhesion sites whose origin could be traced to a position along a filopodium. (B and C) Video frames of selected regions taken from the cell in A showing the appearance of focal complexes along filopodia (arrowheads; see Video 9). Small arrows in C indicate development of filopodia into bundles in the lamella, with focal adhesions at their ends. (D) Video frames of a cell transfected as in A and showing the bending of filopodia (marked 1–3) around adhesion foci (arrowheads). Times are given in minutes and seconds. Bars: (A) 10 μm; (B–D) 5 μm.
Figure 9.
Contribution of microspikes in B16 melanoma cells to construction of actin bundles in the lamella network. (A–C) Video sequences of cells expressing GFP-fascin and mCherry-actin showing transition of fascin-positive microspikes (arrows) into radial (A and B) and transverse bundles (C, arcs) in the lamella. (D) Inhibition of myosin contractility by 30 μM blebbistatin in a B16 melanoma cell expressing GFP-fascin and mCherry-actin (see Video 10). The fascin images were obtained only at the given time points shown in the video sequence to avoid inactivation of blebbistastin. The arc-shaped bundles arising from the lateral translation of microspikes (arrows at time 0:00) dispersed in blebbistatin, but microspike segments continued to enter the lamella (times 15:00 and 40:00). Times are given in minutes and seconds.
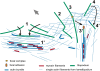
Figure 10.
Schematic illustration of the different fates of filopodia. Large arrow indicates direction of movement. (1 and 1′) Filopodia fold laterally in opposite directions into the cell edge, forming a bundle with antiparallel filaments that integrates into the lamella as the cell edge advances. Integration is coupled with the incorporation of myosin and actin cross-linkers into the bundle. Single filaments originating from the lamellipodium can also contribute to these bundles. (2) A few filopodia kink as a result of lamellipodial retrograde flow and can release fragments into the lamella. (3 and 3′). An early adhesion (focal complex) forms underneath a filopodium. The part of the filopodium distal to the adhesion retracts or folds away, finally separating from the point of adhesion. The proximal part of the filopodium links with oppositely polarized filaments in the lamella via interaction with myosin and actin cross-linkers, forming a contractile stress fiber and resulting in the maturation of the focal complex into a focal adhesion. Again, filaments originating from the lamellipodium can potentially be recruited into the bundle. (4 and 4′). A filopodium folds up and back into the lamella.
Similar articles
- Retrograde flow and myosin II activity within the leading cell edge deliver F-actin to the lamella to seed the formation of graded polarity actomyosin II filament bundles in migrating fibroblasts.
Anderson TW, Vaughan AN, Cramer LP. Anderson TW, et al. Mol Biol Cell. 2008 Nov;19(11):5006-18. doi: 10.1091/mbc.e08-01-0034. Epub 2008 Sep 17. Mol Biol Cell. 2008. PMID: 18799629 Free PMC article. - One step ahead: role of filopodia in adhesion formation during cell migration of keratinocytes.
Schäfer C, Borm B, Born S, Möhl C, Eibl EM, Hoffmann B. Schäfer C, et al. Exp Cell Res. 2009 Apr 15;315(7):1212-24. doi: 10.1016/j.yexcr.2008.11.008. Epub 2008 Dec 3. Exp Cell Res. 2009. PMID: 19100734 - A role for actin arcs in the leading-edge advance of migrating cells.
Burnette DT, Manley S, Sengupta P, Sougrat R, Davidson MW, Kachar B, Lippincott-Schwartz J. Burnette DT, et al. Nat Cell Biol. 2011 Apr;13(4):371-81. doi: 10.1038/ncb2205. Epub 2011 Mar 20. Nat Cell Biol. 2011. PMID: 21423177 Free PMC article. - The comings and goings of actin: coupling protrusion and retraction in cell motility.
Small JV, Resch GP. Small JV, et al. Curr Opin Cell Biol. 2005 Oct;17(5):517-23. doi: 10.1016/j.ceb.2005.08.004. Curr Opin Cell Biol. 2005. PMID: 16099152 Review. - Regulation of actin assembly associated with protrusion and adhesion in cell migration.
Le Clainche C, Carlier MF. Le Clainche C, et al. Physiol Rev. 2008 Apr;88(2):489-513. doi: 10.1152/physrev.00021.2007. Physiol Rev. 2008. PMID: 18391171 Review.
Cited by
- Actin stress fibre subtypes in mesenchymal-migrating cells.
Vallenius T. Vallenius T. Open Biol. 2013 Jun 19;3(6):130001. doi: 10.1098/rsob.130001. Open Biol. 2013. PMID: 23782578 Free PMC article. Review. - The WAVE complex forms linear arrays at negative membrane curvature to instruct lamellipodia formation.
Wu M, Marchando P, Meyer K, Tang Z, Woolfson DN, Weiner OD. Wu M, et al. bioRxiv [Preprint]. 2024 Jul 8:2024.07.08.600855. doi: 10.1101/2024.07.08.600855. bioRxiv. 2024. PMID: 39026726 Free PMC article. Preprint. - Drosophila Fascin is a novel downstream target of prostaglandin signaling during actin remodeling.
Groen CM, Spracklen AJ, Fagan TN, Tootle TL. Groen CM, et al. Mol Biol Cell. 2012 Dec;23(23):4567-78. doi: 10.1091/mbc.E12-05-0417. Epub 2012 Oct 10. Mol Biol Cell. 2012. PMID: 23051736 Free PMC article. - Arp2/3 complex activity in filopodia of spreading cells.
Johnston SA, Bramble JP, Yeung CL, Mendes PM, Machesky LM. Johnston SA, et al. BMC Cell Biol. 2008 Dec 9;9:65. doi: 10.1186/1471-2121-9-65. BMC Cell Biol. 2008. PMID: 19068115 Free PMC article. - Differential effects of tissue culture coating substrates on prostate cancer cell adherence, morphology and behavior.
Liberio MS, Sadowski MC, Soekmadji C, Davis RA, Nelson CC. Liberio MS, et al. PLoS One. 2014 Nov 6;9(11):e112122. doi: 10.1371/journal.pone.0112122. eCollection 2014. PLoS One. 2014. PMID: 25375165 Free PMC article.
References
- Abercrombie, M., J.E. Heaysman, and S.M. Pegrum. 1970. a. The locomotion of fibroblasts in culture. I. Movements of the leading edge. Exp. Cell Res. 59:393–398. - PubMed
- Abercrombie, M., J.E. Heaysman, and S.M. Pegrum. 1970. b. The locomotion of fibroblasts in culture. II. “Ruffling”. Exp. Cell Res. 60:437–444. - PubMed
- Adams, J.C. 1995. Formation of stable microspikes containing actin and the 55 kDa actin bundling protein, fascin, is a consequence of cell adhesion to thrombospondin-1: implications for the anti-adhesive activities of thrombospondin-1. J. Cell Sci. 108:1977–1990. - PubMed
- Adams, J.C. 2004. Roles of fascin in cell adhesion and motility. Curr. Opin. Cell Biol. 16:590–596. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases