Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer - PubMed (original) (raw)
Clinical Trial
Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer
Shengming Dai et al. Mol Ther. 2008 Apr.
Abstract
Exosomes are small membrane vesicles that are secreted by a multitude of cell types. The exosomes derived from dendritic cells (Dex), tumor cells (Tex), and malignant effusions demonstrate immunomodulatory functions, and are even under clinical trial for cancer treatments. In this study we report the phase I clinical trial of the ascites-derived exosomes (Aex) in combination with the granulocyte-macrophage colony-stimulating factor (GM-CSF) in the immunotherapy of colorectal cancer (CRC). The Aex isolated by sucrose/D(2)O density gradient ultracentrifugation are 60-90-nm vesicles that contain the diverse immunomodulatory markers of exosomes and tumor-associated carcinoembryonic antigen (CEA). Totally 40 patients (HLA-A0201(+)CEA(+)) with advanced CRC were enrolled in the study, and randomly assigned to treatments with Aex alone or Aex plus GM-CSF. Patients in both groups received a total of four subcutaneous immunizations at weekly intervals. We found that both therapies were safe and well tolerated, and that Aex plus GM-CSF but not Aex alone can induce beneficial tumor-specific antitumor cytotoxic T lymphocyte (CTL) response. Therefore, our study suggests that the immunotherapy of CRC with Aex in combination with GM-CSF is feasible and safe, and thus can serve as an alternative choice in the immunotherapy of advanced CRC.
Figures
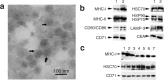
Figure 1
Characterization of Aex. (a) Electron microscopy assay of the isolated Aex. The data were representative of Aex derived from patient H5. Bar = 100 nm. (b) Western blot assay of the protein markers in Aex derived from patient H5. Thirty microgram of cell lysates derived from the ascites (lane 1) or Aex (lane 2) were separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel and examined by Western blot. (c) Western blot assay of major histocompatibility complex class I (MHC-I), HSC70, and CD71 contained in Aex derived from representative patients. Lanes 1–7 correspond to Aex (30 μg per lane) derived from patients A1, B1, C1, D1, E1, F1, and G1, respectively.
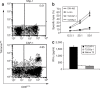
Figure 2
Induction of carcinoembryonic antigen (CEA)-specific cytotoxic T lymphocyte (CTL) by the treatments. (a) Tetramer tests. Representative results derived from triplicate samples were shown. Delayed-type hypersensitivity (DTH) sites–derived emigrated cells were stained with phycoerythrin-labeled HLA-A*0201 tetramers (CAP-1 or SSp-1-specific) and fluorescein isothiocyanate (FITC)-conjugated CD8 monoclonal antibody and finally analyzed by flow cytometry. Numbers indicated for percentages of cells with positive staining. (b) Cytotoxicity assay. DTH site–derived cultured cells were cocultured with 51Cr-labeled SW480 cells, LoVo cells, T2 cells pulsed with CAP-1 (T2/CAP-1), or SSp-1 (T2/SSp-1) peptide. Specific cytotoxicity was evaluated by 51Cr release assay. Results were presented as the mean percentage of specific lysis ± SD of triplicate samples. (c) Interferon-γ (IFN-γ) release assay. CD8+ T cells isolated from the DTH site–derived cultured cells were cocultured with native T2 cells or T2/CAP-1 or T2/SSp-1 for 24 hours. IFN-γ level in the culture supernatant was determined by enzyme-linked immunosorbent assay, and the results were presented as mean ± SD of triplicate samples. Data presented here were the results derived from patient G1 who was treated with 300 μg Aex plus 50 μg granulocyte–macrophage colony-stimulating factor.
Similar articles
- Durable carcinoembryonic antigen (CEA)-specific humoral and cellular immune responses in colorectal carcinoma patients vaccinated with recombinant CEA and granulocyte/macrophage colony-stimulating factor.
Ullenhag GJ, Frödin JE, Jeddi-Tehrani M, Lidströmer N, Strigård K, Eriksson E, Samanci A, Choudhury A, Nilsson B, Rossmann ED, Mosolits S, Mellstedt H. Ullenhag GJ, et al. Clin Cancer Res. 2004 May 15;10(10):3273-81. doi: 10.1158/1078-0432.CCR-03-0706. Clin Cancer Res. 2004. PMID: 15161680 Clinical Trial. - The influence of granulocyte macrophage colony-stimulating factor and prior chemotherapy on the immunological response to a vaccine (ALVAC-CEA B7.1) in patients with metastatic carcinoma.
von Mehren M, Arlen P, Gulley J, Rogatko A, Cooper HS, Meropol NJ, Alpaugh RK, Davey M, McLaughlin S, Beard MT, Tsang KY, Schlom J, Weiner LM. von Mehren M, et al. Clin Cancer Res. 2001 May;7(5):1181-91. Clin Cancer Res. 2001. PMID: 11350882 Clinical Trial. - Induction of G250-targeted and T-cell-mediated antitumor activity against renal cell carcinoma using a chimeric fusion protein consisting of G250 and granulocyte/monocyte-colony stimulating factor.
Tso CL, Zisman A, Pantuck A, Calilliw R, Hernandez JM, Paik S, Nguyen D, Gitlitz B, Shintaku PI, de Kernion J, Figlin R, Belldegrun A. Tso CL, et al. Cancer Res. 2001 Nov 1;61(21):7925-33. Cancer Res. 2001. PMID: 11691814 - Recent progress in GM-CSF-based cancer immunotherapy.
Yan WL, Shen KY, Tien CY, Chen YA, Liu SJ. Yan WL, et al. Immunotherapy. 2017 Mar;9(4):347-360. doi: 10.2217/imt-2016-0141. Immunotherapy. 2017. PMID: 28303764 Review.
Cited by
- Engineering Extracellular Vesicles for Cancer Therapy.
Nedeva C, Mathivanan S. Nedeva C, et al. Subcell Biochem. 2021;97:375-392. doi: 10.1007/978-3-030-67171-6_14. Subcell Biochem. 2021. PMID: 33779924 - Targeted Delivery of Mesenchymal Stem Cell-Derived Nanovesicles for Spinal Cord Injury Treatment.
Lee JR, Kyung JW, Kumar H, Kwon SP, Song SY, Han IB, Kim BS. Lee JR, et al. Int J Mol Sci. 2020 Jun 11;21(11):4185. doi: 10.3390/ijms21114185. Int J Mol Sci. 2020. PMID: 32545361 Free PMC article. - Exosomes as mediators of immune regulation and immunotherapy in cancer.
Kugeratski FG, Kalluri R. Kugeratski FG, et al. FEBS J. 2021 Jan;288(1):10-35. doi: 10.1111/febs.15558. Epub 2020 Oct 3. FEBS J. 2021. PMID: 32910536 Free PMC article. Review. - Medulloblastoma exosome proteomics yield functional roles for extracellular vesicles.
Epple LM, Griffiths SG, Dechkovskaia AM, Dusto NL, White J, Ouellette RJ, Anchordoquy TJ, Bemis LT, Graner MW. Epple LM, et al. PLoS One. 2012;7(7):e42064. doi: 10.1371/journal.pone.0042064. Epub 2012 Jul 27. PLoS One. 2012. PMID: 22848702 Free PMC article. - RAFTsomes containing epitope-MHC-II complexes mediated CD4+ T cell activation and antigen-specific immune responses.
Ding Q, Chen J, Wei X, Sun W, Mai J, Yang Y, Xu Y. Ding Q, et al. Pharm Res. 2013 Jan;30(1):60-9. doi: 10.1007/s11095-012-0849-7. Epub 2012 Aug 10. Pharm Res. 2013. PMID: 22878683
References
- Keller S, Sanderson MP, Stoeck A, Altevogt P. Exosomes: from biogenesis and secretion to biological function. Immunol Lett. 2006;107:102–108. - PubMed
- Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. - PubMed
- Chaput N, Flament C, Viaud S, Taieb J, Roux S, Spatz A. Dendritic cell derived-exosomes: biology and clinical implementations. J Leukoc Biol. 2006;80:471–478. - PubMed
- Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4:594–600. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials